Ebastine solid dispersion and high-dissolubility ebastine tablet prepared by the same
A technology of solid dispersion and ebastine tablets, which is applied in the field of medicine, can solve problems such as insoluble water, low bioavailability, and poor dissolution rate of tablets, and achieve improved solubility, no significant change, and stable dissolution rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 (blank control)
[0028] prescription
[0029] Ebastine 5.7%
[0030] Lactose 51.4%
[0031] Starch 28.6%
[0032] Punch starch 11.4%
[0033] Low-substituted hydroxypropyl cellulose 2.9%
[0034] Magnesium Stearate 0.1%
[0035] method
[0036] Weigh the pulping starch according to the prescription, use appropriate amount of water to make 12% starch slurry, and weigh lactose, starch, and low-substituted hydroxypropyl cellulose according to the prescription ratio, crush them separately, pass through a 100-mesh sieve, mix, and add 12% Starch slurry, made into soft material, granulated with a 16-mesh sieve, dried at 55-60°C, magnesium stearate added to the dry granules, granulated with a 14-mesh sieve, mixed, compressed into tablets, packed in aluminum and aluminum.
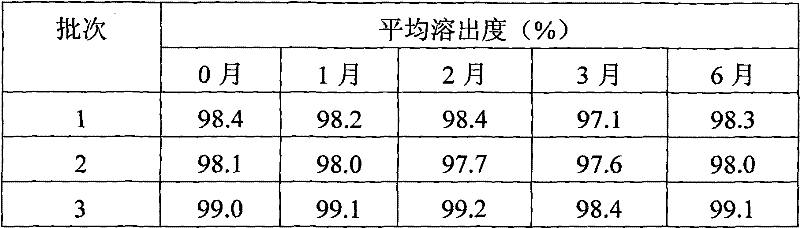
[0037] Dissolution test results
[0038] film number
Embodiment 2
[0040] prescription
[0041] Ebastine 5.7%
[0042] Lactose 45.7%
[0043] Starch 22.9%
[0044] Punch starch 11.4%
[0045] Low-substituted hydroxypropyl cellulose 2.9%
[0046] Poloxamer F68 11.4%
[0047] Magnesium Stearate 0.1%
[0048] method
[0049]Weigh ebastine and loxamer F68 according to the prescription ratio, add medicinal ethanol, stir, heat to 60°C, fully mix, continue to stir for 1 hour, evaporate the solvent under reduced pressure at 65°C, cool to room temperature, Filtrate and dry in vacuum at 70°C to obtain solid dispersions of ebastine and poloxamer F68; weigh the pulped starch according to the prescription, and make 12% starch slurry with an appropriate amount of water; weigh lactose and starch according to the ratio of the prescription , low-substituted hydroxypropyl cellulose, solid dispersion of ebastine and poloxamer F68, respectively crushed, passed through a 100-mesh sieve, mixed, added 12% starch slurry, made into a soft material, and granula...
Embodiment 3
[0053] prescription
[0054] Ebastine 5.7%
[0055] Lactose 40.0%
[0056] Starch 17.1%
[0057] Punch starch 11.4%
[0058] Low-substituted hydroxypropyl cellulose 2.9%
[0059] Poloxamer F68 22.8%
[0060] Magnesium Stearate 0.1%
[0061] method
[0062] Weigh ebastine and loxamer F68 according to the prescription ratio, add medicinal ethanol, stir, heat to 40°C, fully mix, continue to stir for 2 hours, evaporate the solvent under reduced pressure at 45°C, cool to room temperature, Filtrate and dry under vacuum at 50°C to obtain solid dispersions of ebastine and poloxamer F68; weigh the starch for pulping according to the prescription, and make 12% starch slurry with an appropriate amount of water; weigh lactose and starch according to the ratio of the prescription , low-substituted hydroxypropyl cellulose, solid dispersion of ebastine and poloxamer F68, respectively crushed, passed through a 100-mesh sieve, mixed, added 12% starch slurry, made into a soft material, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com