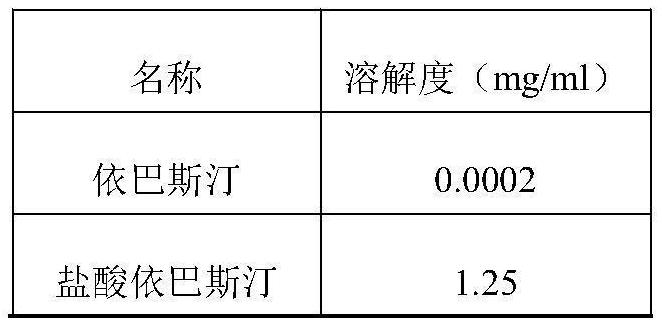
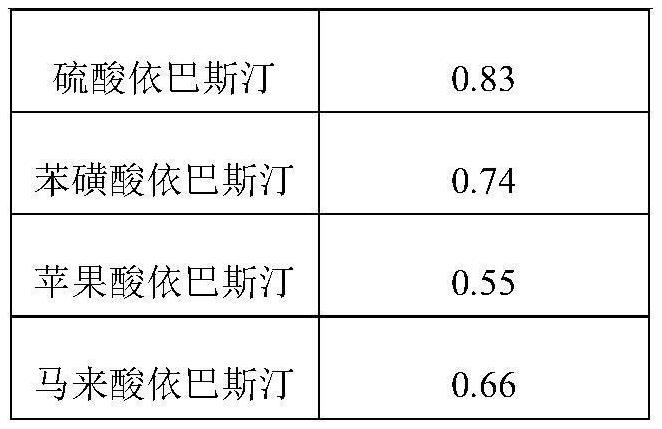
Ebastine salt tablet and preparation method thereof
A technology for ebastine and tablet is applied in the field of ebastine salt tablet and its preparation, which can solve the problems of low solubility, slow dissolution, low bioavailability, etc. The effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of the tablet of the salt of above-mentioned ebastine is as follows:
[0024] Weigh a class of ebastine salt, lactose, starch, and microcrystalline cellulose into a wet granulator, stir, chop, add purified water to make soft materials, wet and granulate by a granulator, and use a fluidized granulator. The bed is dried. The dry granules are mixed with magnesium stearate and croscarmellose sodium to obtain intermediate granules. The intermediate particles are taken for testing, and after passing the inspection, the intermediate particles are compressed to obtain intermediate voxel tablets. The intermediate voxel tablets were taken for testing, and after passing the inspection, the intermediate voxel tablets were coated to obtain intermediate-coated tablets. Take the intermediate-coated tablets for testing, and after passing the inspection, aluminum-plastic and outsourcing the intermediate-coated tablets.
[0025] The above-mentioned inspection pr...
Embodiment 1
[0043] Table 3. Ebastine Hydrochloride Tablet Formulation
[0044]
[0045] Preparation:
[0046] Weigh ebastine hydrochloride, lactose, starch, and microcrystalline cellulose into a wet granulator, stir, chop, add purified water to make soft materials, wet and granulate by a granulator, and use a fluidized bed for drying . The dry granules are mixed with magnesium stearate and croscarmellose sodium to obtain intermediate granules. The intermediate particles are taken for testing, and after passing the inspection, the intermediate particles are compressed to obtain intermediate voxel tablets. The intermediate voxel tablets were taken for testing, and after passing the inspection, the intermediate voxel tablets were coated to obtain intermediate-coated tablets. Take the intermediate-coated tablets for testing, and after passing the inspection, aluminum-plastic and outsourcing the intermediate-coated tablets.
Embodiment 2
[0048] Table 4 Ebastine sulfate tablet prescription
[0049]
[0050]
[0051] Preparation:
[0052] Weigh ebastine sulfate, lactose, starch, and microcrystalline cellulose into a wet granulator, stir, chop, add purified water to make soft materials, wet and granulate by a granulator, and use a fluidized bed for drying . The dry granules are mixed with magnesium stearate and croscarmellose sodium to obtain intermediate granules. The intermediate particles are taken for testing, and after passing the inspection, the intermediate particles are compressed to obtain intermediate voxel tablets. The intermediate voxel tablets were taken for testing, and after passing the inspection, the intermediate voxel tablets were coated to obtain intermediate-coated tablets. Take the intermediate-coated tablets for testing, and after passing the inspection, aluminum-plastic and outsourcing the intermediate-coated tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle of repose | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com