Taste-masked pharmaceutical compositions with gastrosoluble pore-formers
a technology of poreformers and pharmaceutical compositions, which is applied in the direction of microcapsules, capsule delivery, organic active ingredients, etc., can solve the problems of poor taste, negative impact on the efficacy of treatment, and even non-compliance with treatment, so as to enhance the probability of achieving bioequivalence, taste-masking effect, and rapid/complete releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0086] Drug-layered Diphenhydramine hydrochloride Beads (drug load: 15%): Diphenhydramine hydrochloride (375 g) was slowly added to an aqueous solution of 41.8 g polyvinylpyrrolidone (binder) and 1667 g of purified water and mixed well. 60-80 mesh sugar spheres (1470 g) were coated with the drug-layering formulation in a Glatt GPCG 3. The drug containing pellets were dried, and a seal coat of Opadry Clear for a weight gain of 4% was applied on the drug-layered beads.
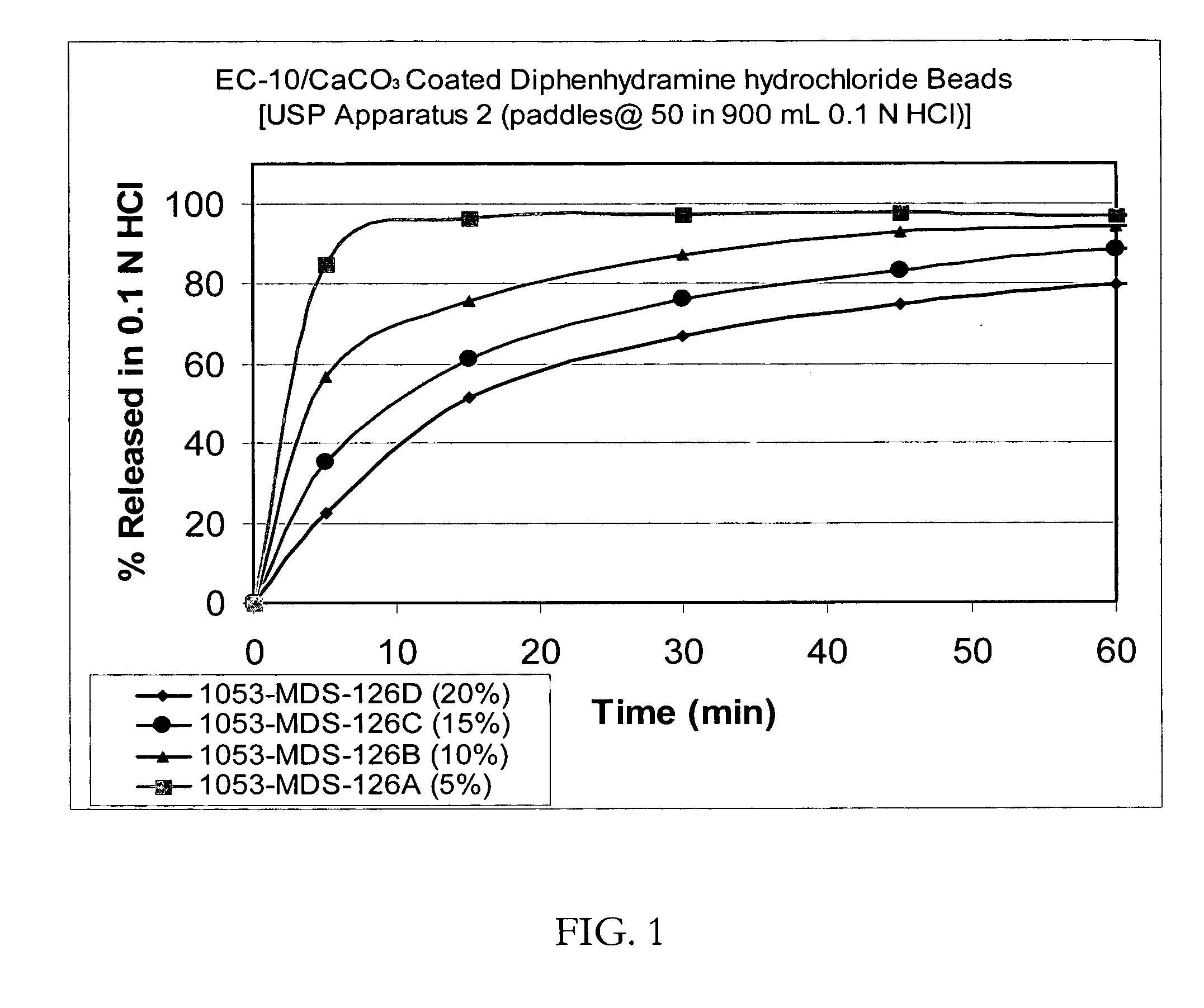
[0087] Taste-masked Beads with Ethylcellulose (EC-10) / Calcium Carbonate: 1000 g of drug-layered beads produced above were coated in the Glatt GPCG 3 with a membrane comprising 227.3 g of EC-10, 22.7 g of Myvacet 9-45 (diacetylated monoglyceride) and 68.2 g of calcium carbonate dissolved / suspended in 3916.6 g of 95 / 5 acetone / water. The coated beads were dried in the Glatt GPCG-3. The dissolution profiles in 0.1N HCl of the beads with a membrane thickness of up to 20% by weight are shown in FIG. 1.
example 2 (
Reference)
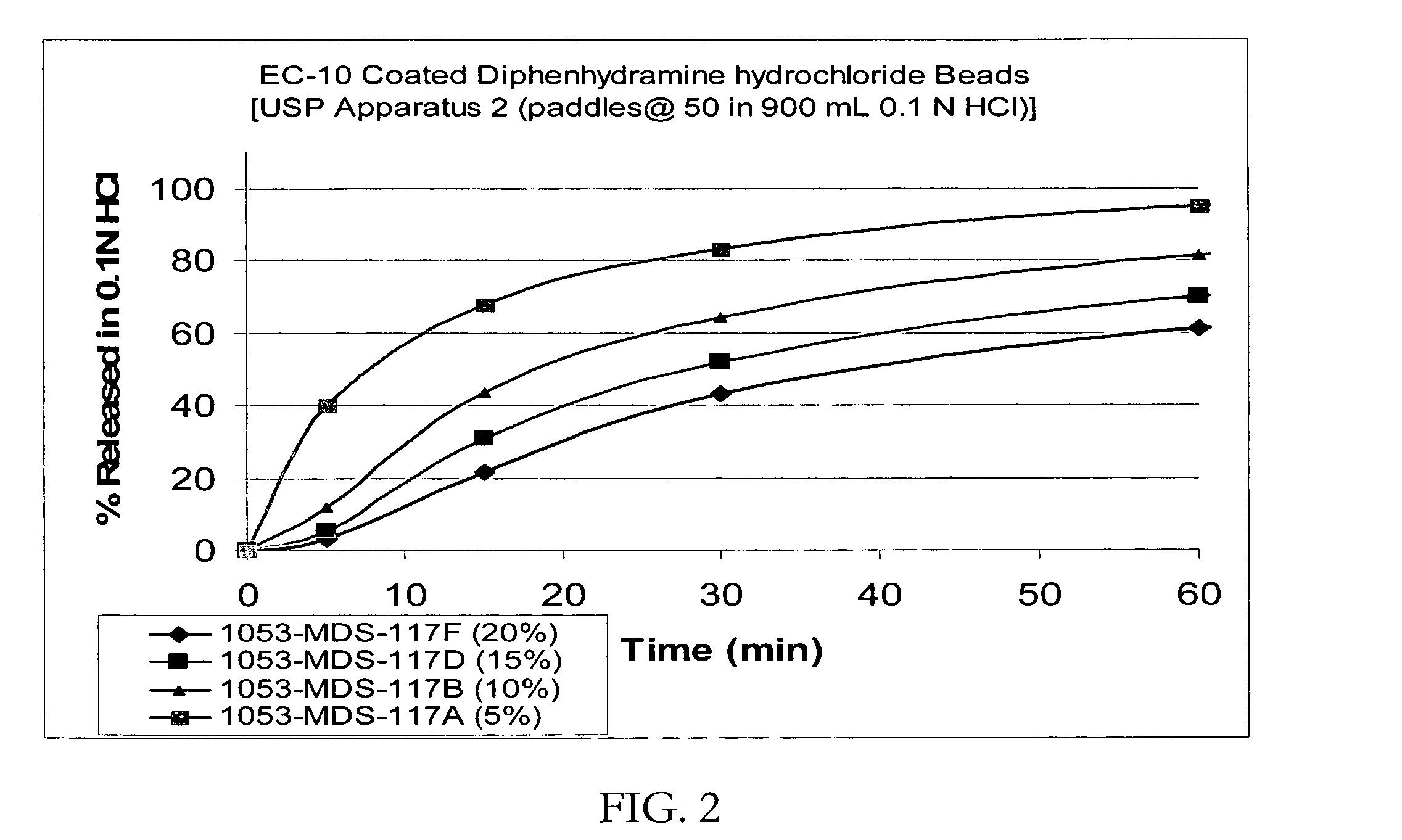
[0088] Taste-masked Beads with Ethylcellulose (EC-10) alone: IR beads were coated with a solution of EC-10 / Myvacet 9-45 at a ratio of 90 / 10 dissolved in 95 / 5 acetone / water for a weight gain of up to 20%. The coated beads were dried in the Glatt GPCG-3. The taste-masked beads coated at 20% typically release less than about 10% in 5 minutes when dissolution tested using the USP Apparatus 2 (paddles @ 50 rpm) in a phosphate buffer at pH 6.8. The dissolution profiles in 0.1N HCl of the beads with a membrane thickness of up to 20% by weight are shown in FIG. 2 suggesting that both taste-masking and rapid release can be achieved when coated with ethylcellulose alone from a solvent mixture although the dissolution profiles from the beads thus coated at acceptable taste-masking levels do not meet the desired dissolution profile for a corresponding immediate release product.
[0089] Rapidly Dispersing Microgranules: The rapidly dispersing microgranules may comprise a sugar alcohol s...
example 3
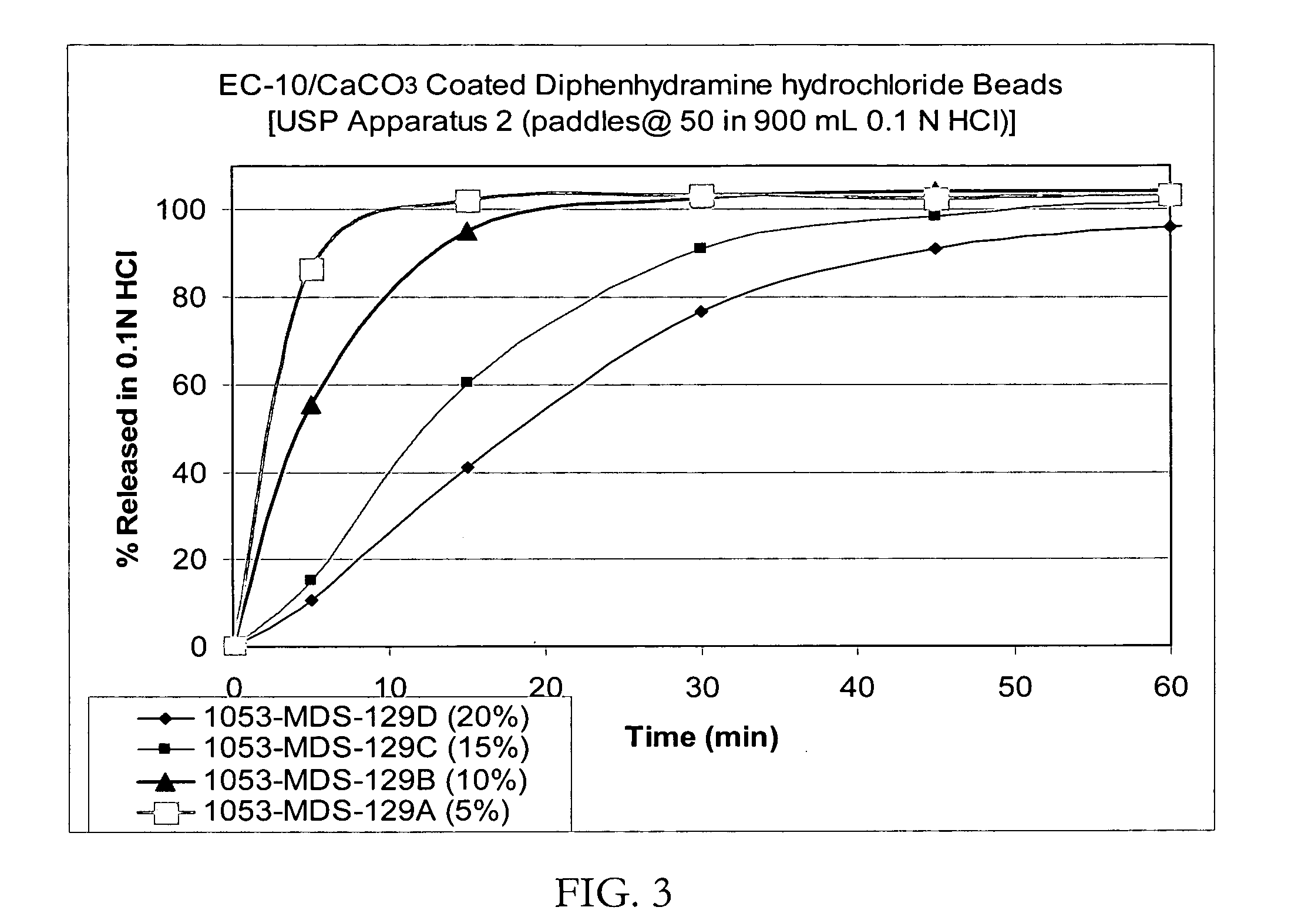
[0091] Taste-masked Beads with Polyvinyl acetate / Calcium Carbonate: 1000 g of drug-layered beads were coated in the Glatt GPCG 3 with a membrane comprising 550 g of Kollicoat SR30D (30% polyvinyl acetate aqueous dispersion), 5.8 g of Myvacet, 49.5 g of micronized calcium carbonate and 30 g of magnesium stearate dissolved / suspended in 2760.9 g of ethanol (final ratio of ethanol / water: 87 / 13). The coated beads were dried in the Glatt GPCG-3. The dissolution profiles in 0.1N HCl of the beads with a membrane thickness of up to 20% by weight are shown in FIG. 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com