Vonoprazan fumarate compound and pharmaceutical composition thereof
A fumarate compound and compound technology, applied in the field of medicinal chemistry, can solve the problems of PPI can not fully inhibit the acid secretion at night, do not provide compound crystal information, can not continuously control the acid secretion, etc. Short, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
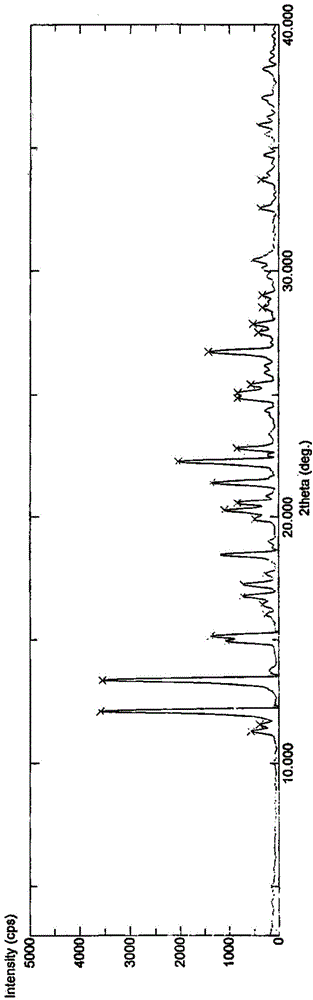
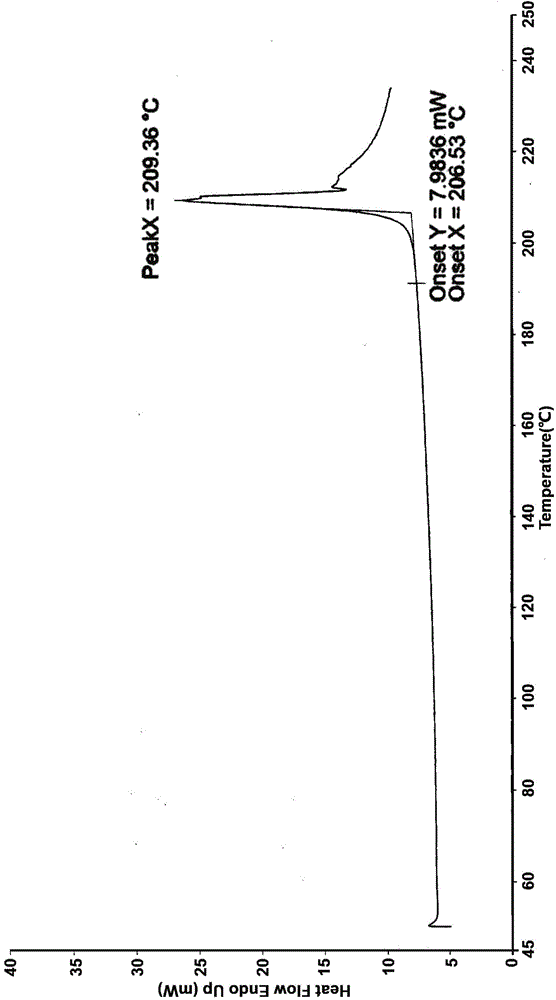
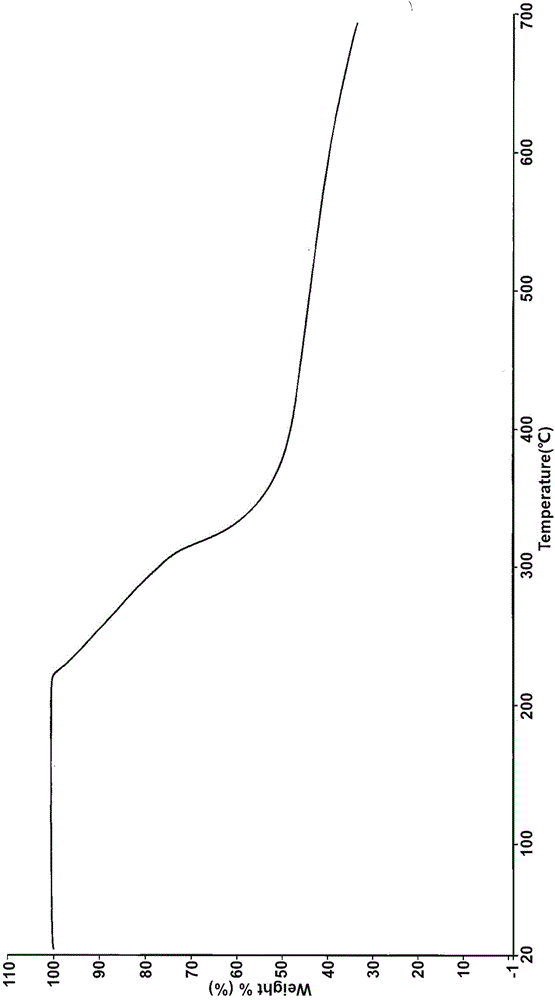
[0035] Suspend the crude product of Vonoprazan fumarate (1g) in a mixed solvent of ethanol / water (3:1, 10mL), heat to dissolve at an internal temperature of 60-65°C, slowly cool down to room temperature, stir for 1h, and then cool down to the internal temperature Stir and crystallize at 0-10°C for 1h, filter, and vacuum-dry at 50°C for 8h to obtain 0.741g of white crystalline powder with a yield of 74.1%.
Embodiment 2
[0037] Suspend the crude Vonoprazan fumarate (1g) in a mixed solvent of ethanol / water (1:1, 15mL), heat to dissolve at an internal temperature of 60-65°C, slowly cool down to room temperature, stir for 1h, and then cool down to the internal temperature Stir and crystallize at 0-10°C for 1h, filter, and vacuum-dry at 50°C for 8h to obtain 0.663g of white crystalline powder with a yield of 66.3%.
Embodiment 3
[0039] Suspend the crude Vonoprazan fumarate (1g) in a mixed solvent of ethanol / water (1:2, 21mL), heat to dissolve at an internal temperature of 50-60°C, slowly cool down to room temperature, stir for 3 hours, and then cool down to the internal temperature Stir and crystallize at 0-10°C for 3h, filter, and vacuum-dry at 40°C for 10h to obtain 0.654g of white crystalline powder with a yield of 65.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com