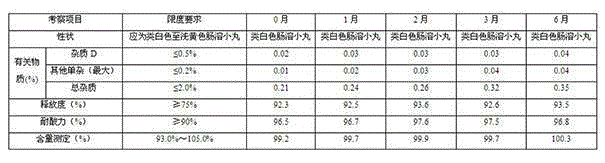
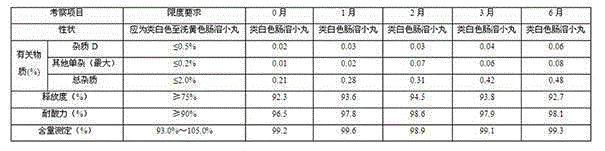
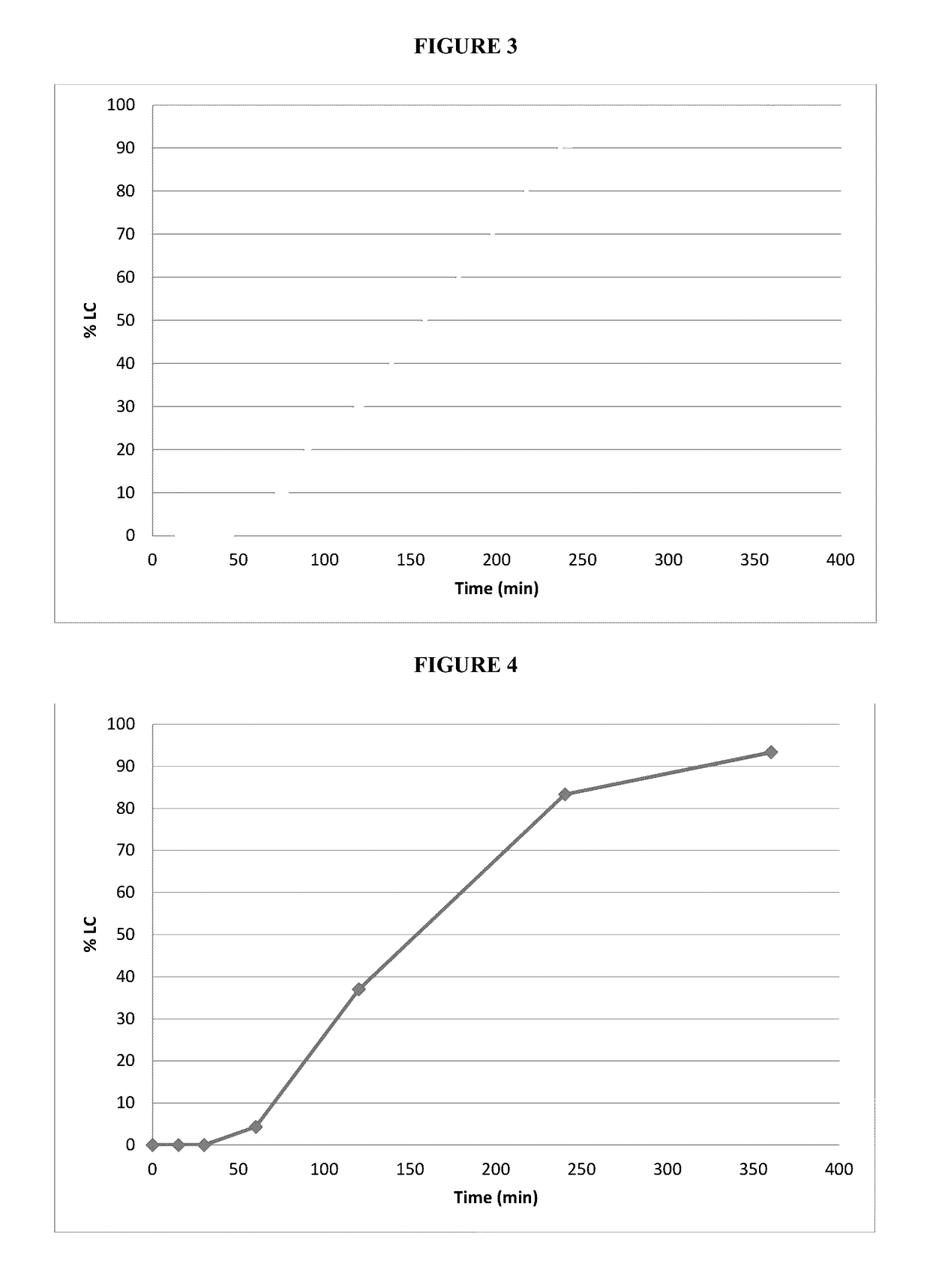
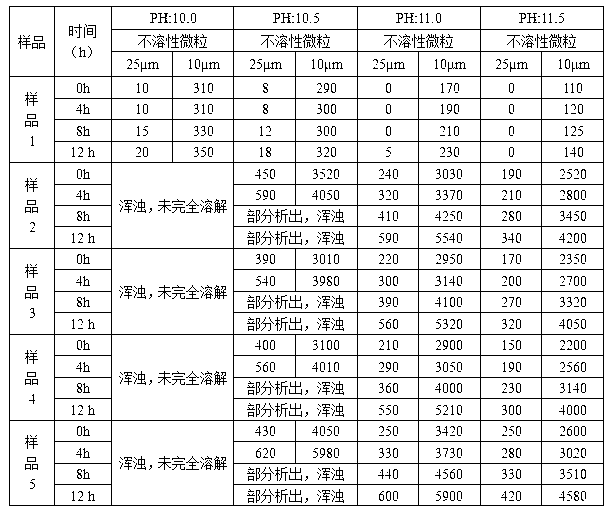
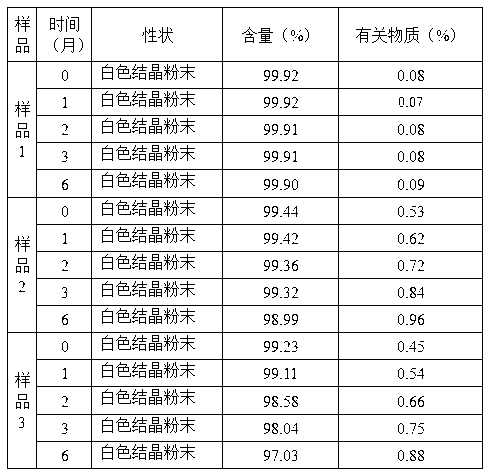
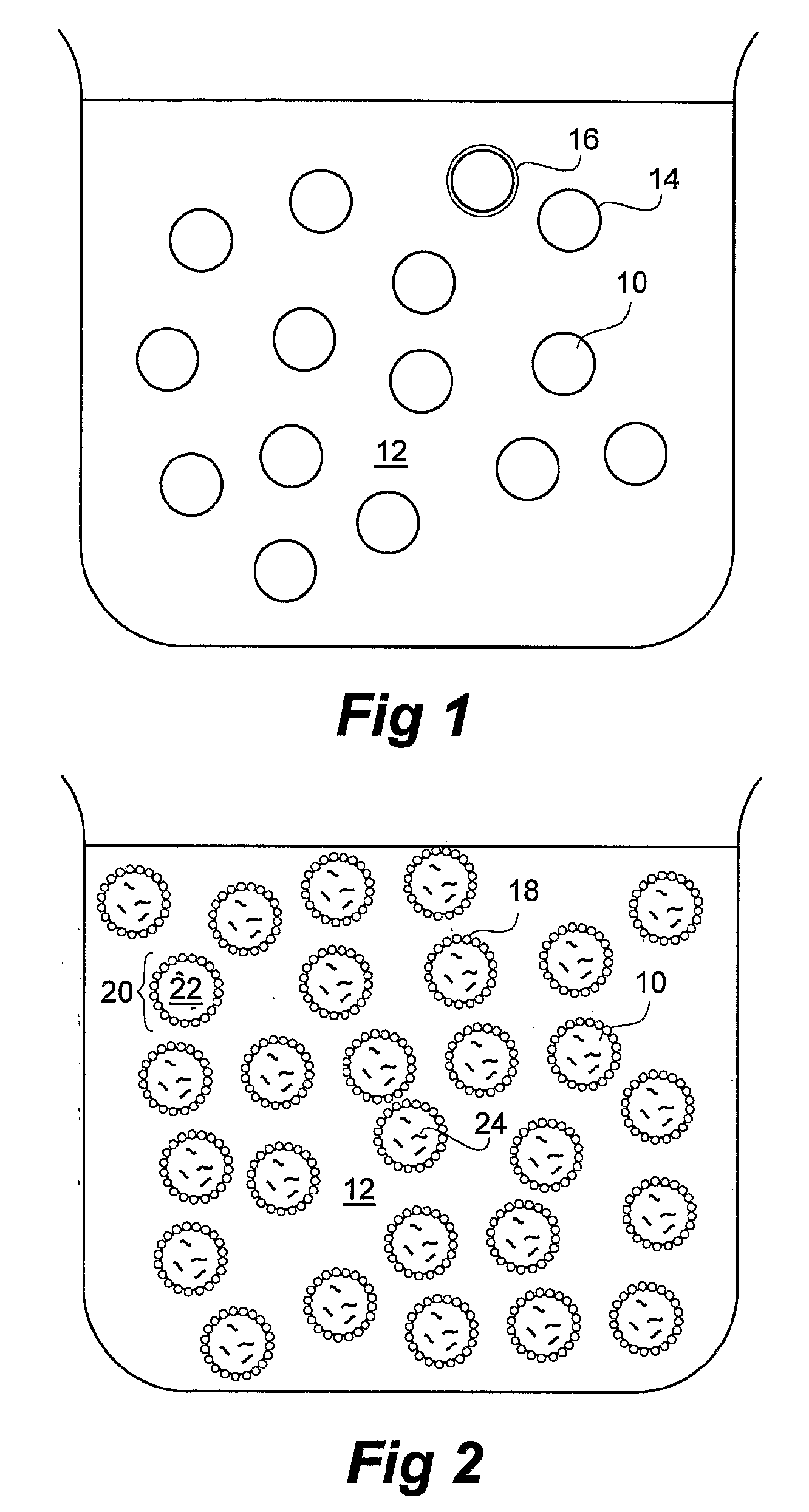
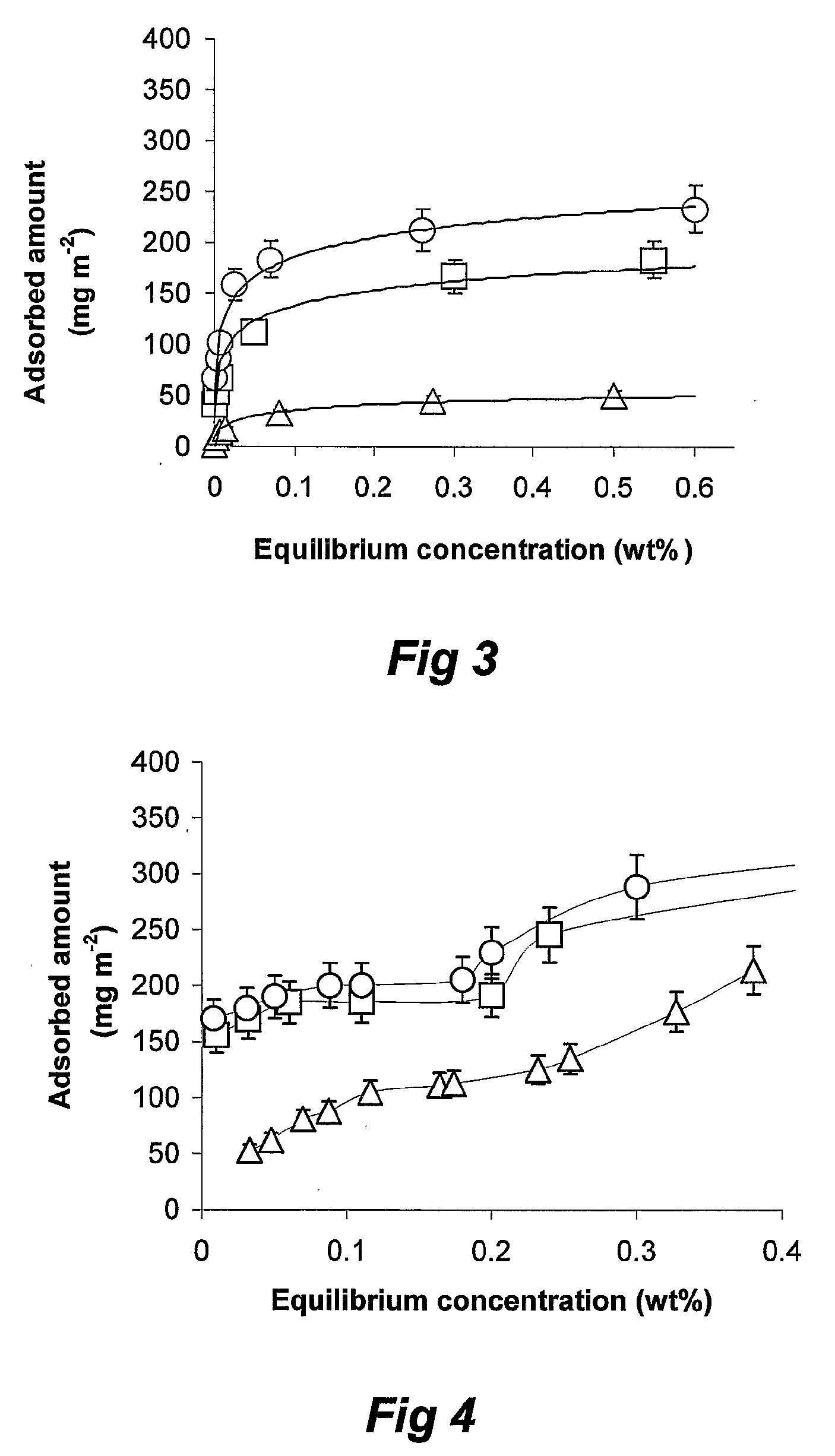
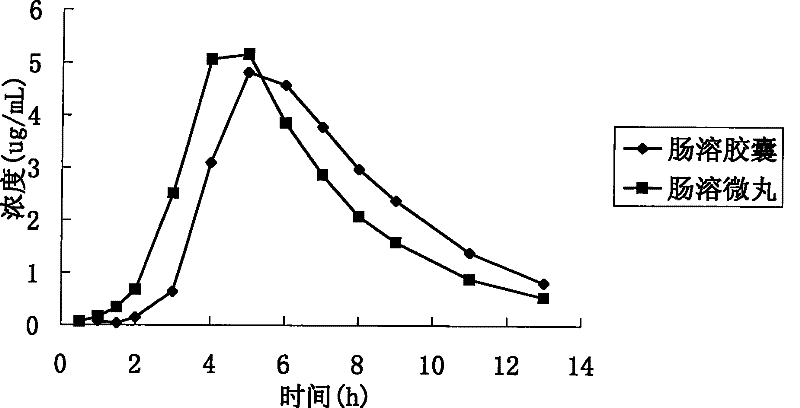
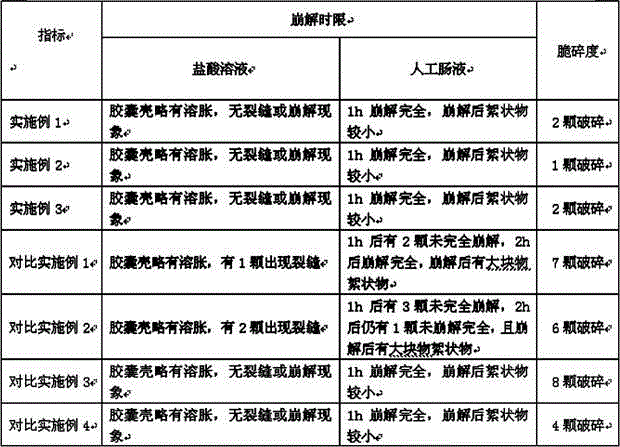
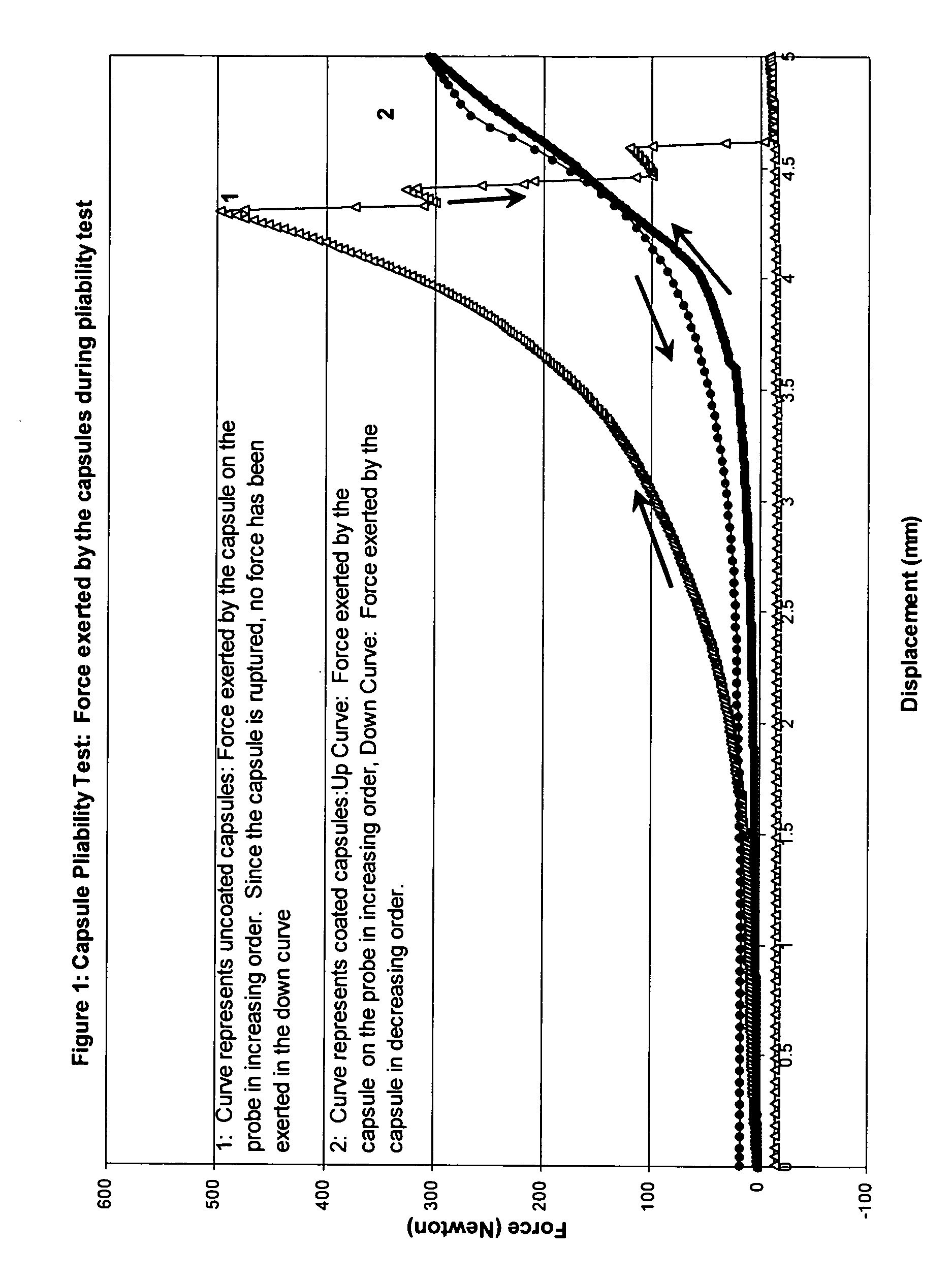
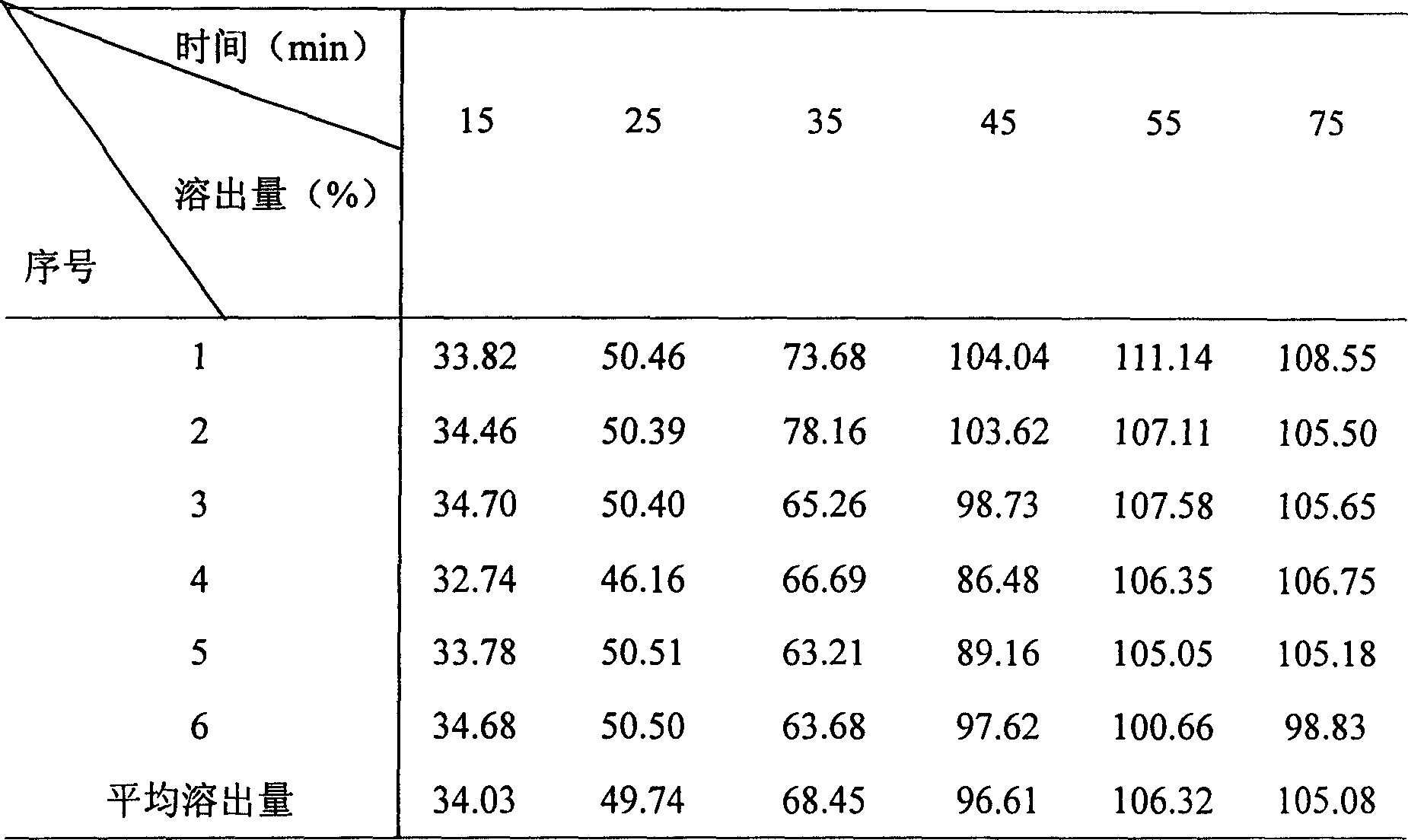
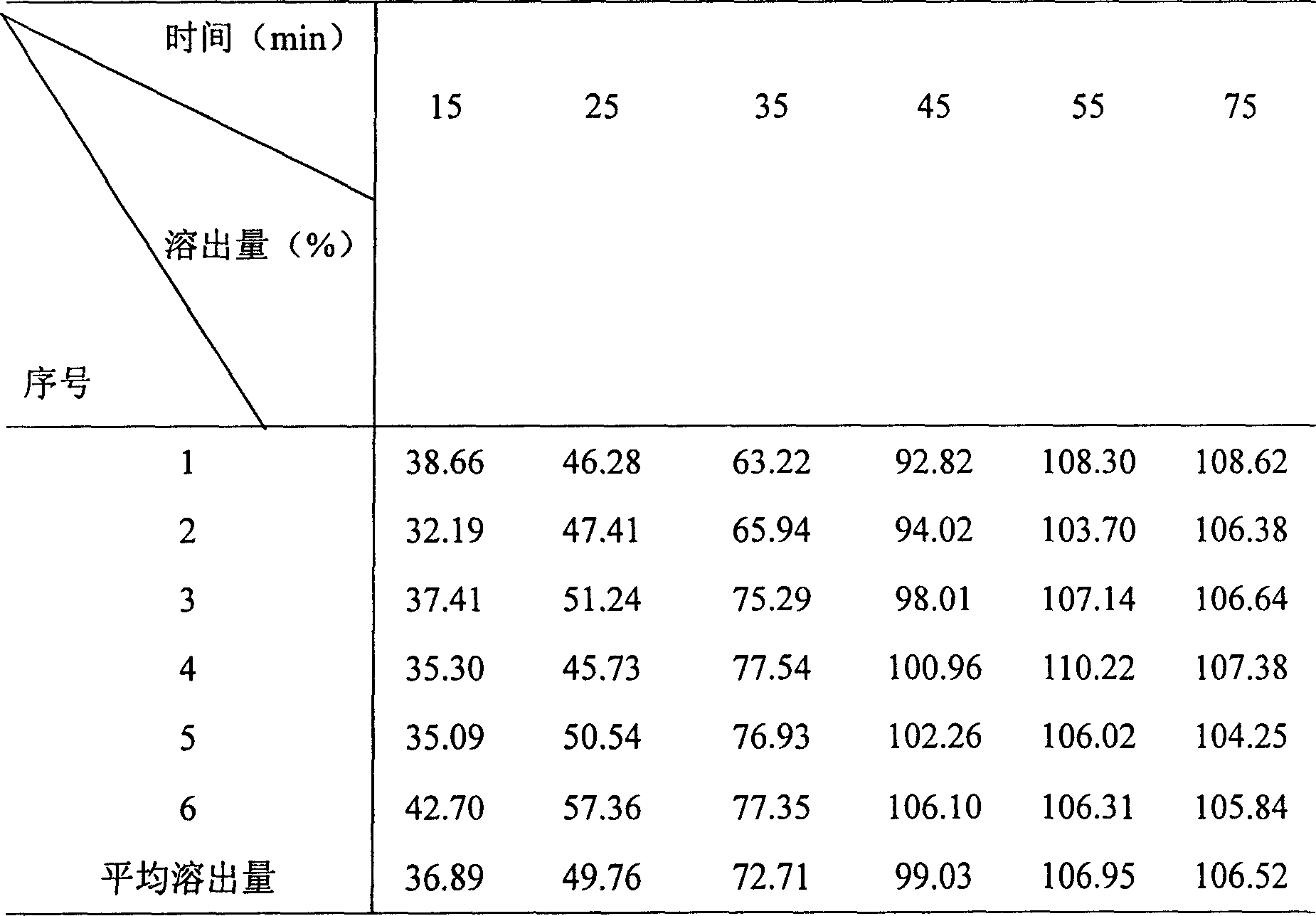
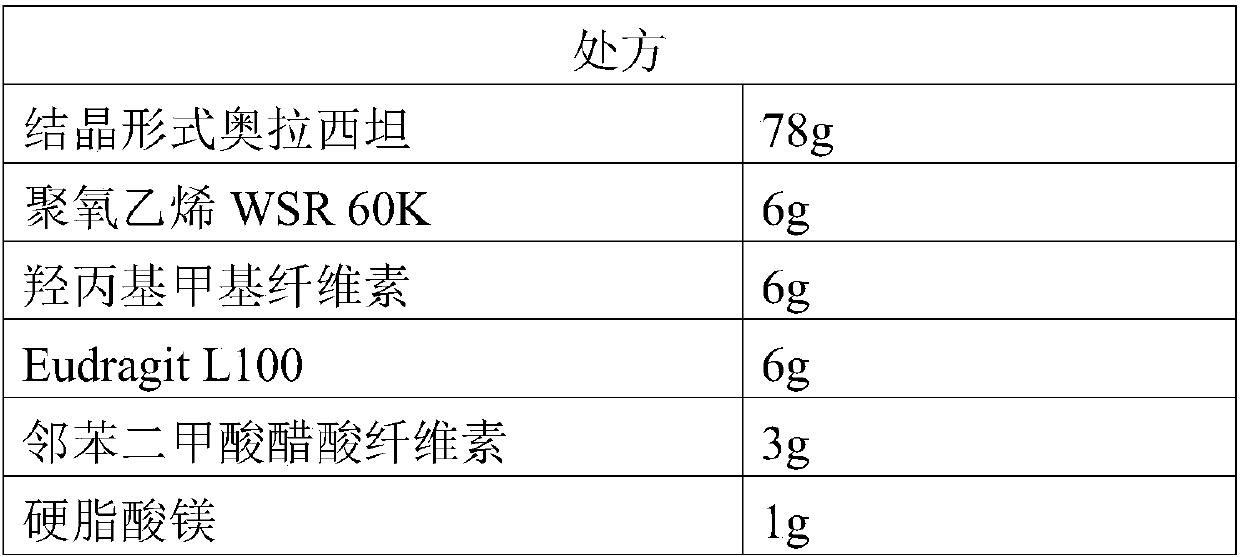
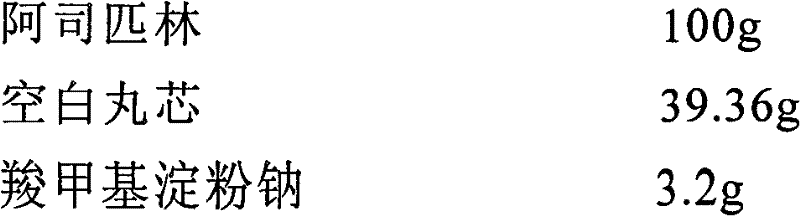Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Coated capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
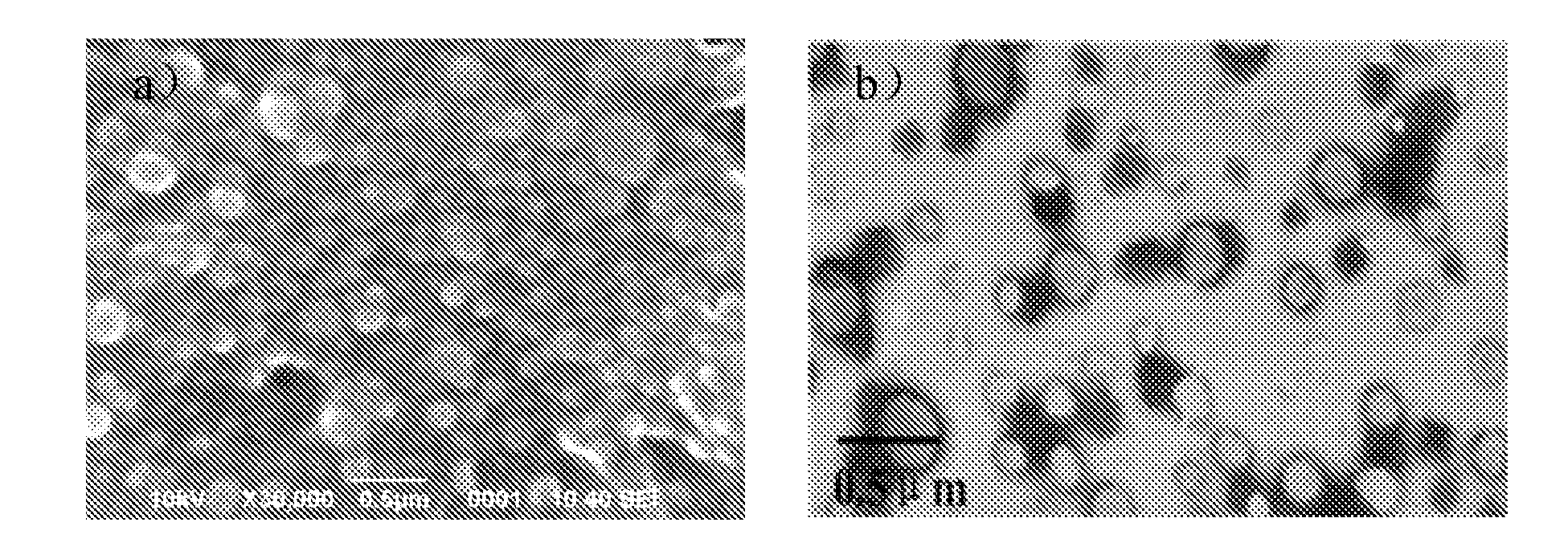
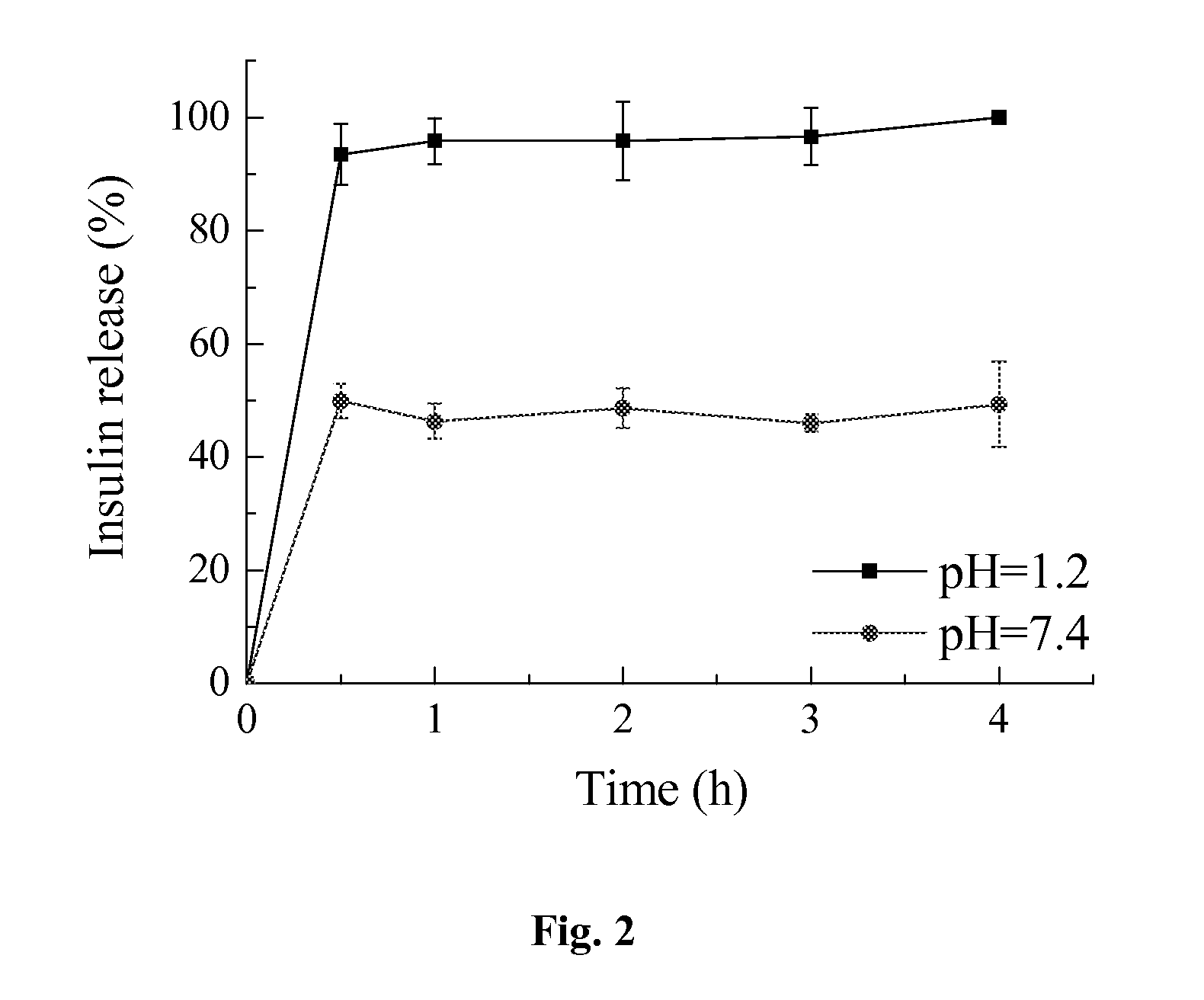
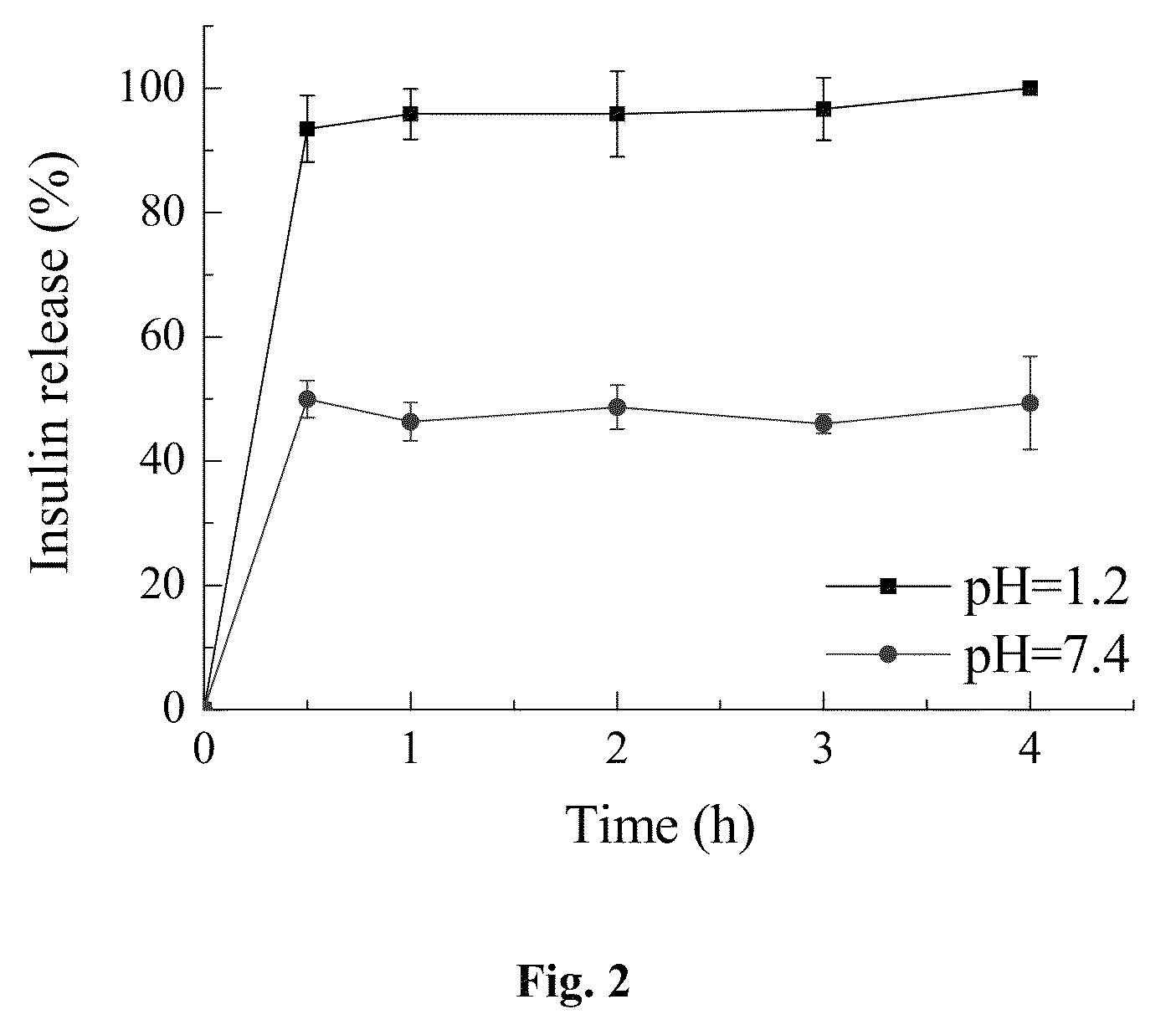
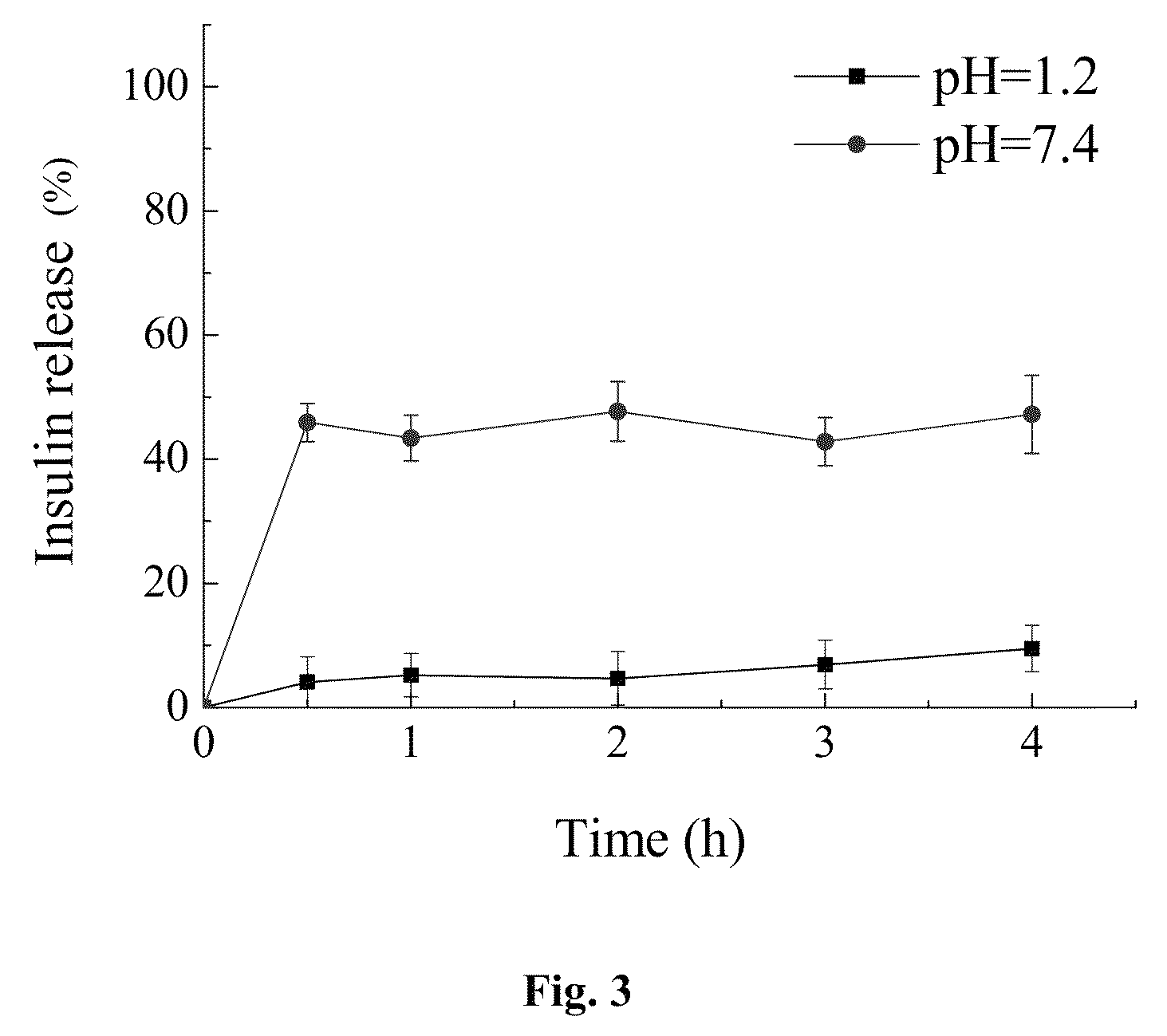
Enteric-coated capsule containing cationic nanoparticles for oral insulin delivery
ActiveUS20130034602A1Enhance absorptionPromote absorptionPowder deliveryPeptide/protein ingredientsMucoadhesive polymersSurface charges
The invention relates to an enteric-coated capsule containing cationic nanoparticles for oral insulin delivery, in particular to a type of cationic nanoparticle including a polycationic and mucoadhesive polymer and a biodegradable polymer, wherein each of the nanoparticles has positive surface charge and enhanced permeability for paracellular insulin delivery; the enteric-coated capsule further includes a pH-sensitive polymer as the coating. The enteric-coated capsule containing cationic nanoparticles, when being orally administered to a subject, are configured to prevent the acidic degradation of the active substance such as insulin before being released from said cationic nanoparticles to a specific absorption site along the gastrointestinal tract.
Owner:NANO & ADVANCED MATERIALS INST
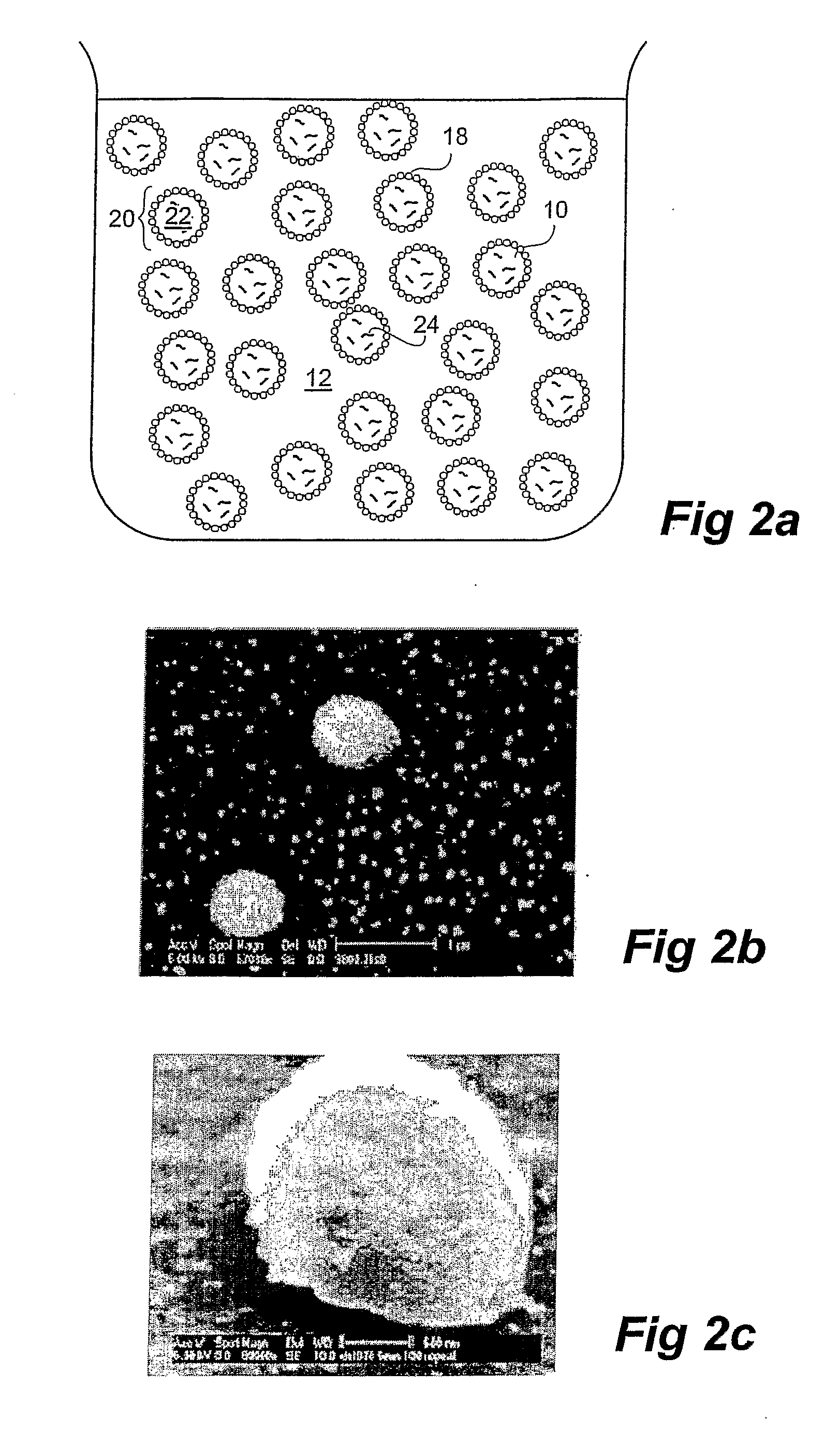
Drug Release From Nanoparticle-Coated Capsules
InactiveUS20110229559A1Enhances congregationDiffusion slowBiocideHydroxy compound active ingredientsSolubilityVitamin A Retinol
Methods of producing a controlled release formulation for an active substance are disclosed, wherein the methods involve dispersing a discontinuous phase comprising an active substance into a continuous phase so as to form a two-phase liquid system comprising droplets of said discontinuous phase, and allowing nanoparticles provided to the two-phase liquid system to congregate at the phase interface to thereby coat the surface of the droplets in at least one layer of said nanoparticles. The methods utilise a concentration of a suitable electrolyte which enhances the nanoparticle congregation such that the coating of nanoparticles on the surface of the droplets presents a semi-permeable barrier to the active substance, or otherwise utilise a amount of the active substance that is greater than the solubility limit of that active substance in the discontinous phase. Formulations comprising vitamin A (retinol) as the active substance for dermal delivery are specifically exemplified.
Owner:UNIV OF SOUTH AUSTRALIA
Drug Release From Nanoparticle-Coated Capsules
InactiveUS20090181076A1Enhances congregationDiffusion slowBiocidePowder deliverySolubilityVitamin A Retinol
Methods of producing a controlled release formulation for an active substance are disclosed, wherein the methods involve dispersing a discontinuous phase comprising an active substance into a continuous phase so as to form a two-phase liquid system comprising droplets of said discontinuous phase, and allowing nanoparticles provided to the two-phase liquid system to congregate at the phase interface to thereby coat the surface of the droplets in at least one layer of said nanoparticles. The methods utilise a concentration of a suitable electrolyte which enhances the nanoparticle congregation such that the coating of nanoparticles on the surface of the droplets presents a semi-permeable barrier to the active substance, or otherwise utilise a amount of the active substance that is greater than the solubility limit of that active substance in the discontinous phase. Formulations comprising vitamin A (retinol) as the active substance for dermal delivery are specifically exemplified.
Owner:SOUTH AUSTRALIA UNIV OF
Aspirin enteric-coated pellet
ActiveCN101596166AEasy accessAvoid stimulationOrganic active ingredientsAntipyreticBlood concentrationBioavailability
The invention discloses an aspirin enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 5-22 blank core pellet, 55-75 medicinal layer consisting of aspirin and medicinal excipients and 20-35 enteric-coated layer. The invention also discloses a method for preparing the aspirin enteric-coated pellet and an aspirin enteric-coated capsule containing the aspirin enteric-coated pellets. The aspirin enteric-coated capsule has the advantages of good stability, small stimulation, steady blood concentration, high bioavailability, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Erythromycin enteric-coated capsule and preparation thereof
ActiveCN101239051ASmooth releaseLittle change in releaseAntibacterial agentsOrganic active ingredientsBiotechnologyMicrobiology
The invention provides an erythromycin enteric-coated capsule and the preparation method. The invention is characterized in that: the component ratio of the enteric-coated liquid of the enteric-pellets capsules contained in the enteric-coated capsules counted by weight percentage is that: polyacrylic resin: II: 1, diethyl phthalate: 0.07 to 0.1, Tween-80:0.08 to 0.12, castor oil: 0.08 to 0.12, ethanol whose concentration is bigger than or equal to 95%: 12 to 14; all are counted as weight percentage. The invention conquers defects in the prior art, elevates the product quality, keeps the releasing degree of the product stable and guarantees the quality as well as healing effect of the product during the period of validity, and also the preparation is simple and convenient, the work efficiency is high and the quality of the products is quite well.
Owner:TIANSHENG PHARMA GROUP
Method for spraying warning mark on surface of airplane heating part or equipment component
ActiveCN102029248AAvoid burnsPretreated surfacesThermometers using physical/chemical changesEngineeringColor changes
The invention discloses a method for spraying a warning mark on the surface of an airplane heating part or an equipment component. The method comprises the following steps of: 1) treating the surface of an airplane on which the warning mark is to be sprayed so as to form a character surface on the surface of the airplane and form a plurality of colored recesses on the character surface; 2) spraying an encapsulated reversible temperature sensing and color change paint on the character surface so as to fill paint-coated capsule particles in the colored recesses on the character surface; 3) sintering the character surface; 4) coating a protective film layer on the sintered character surface; and 5) resintering the character surface and smoothing the coated character surface after the resintering. By the method, a sprayed warning character can directly reflect the surface temperature state of the airplane heating part or the equipment component, so that operation personnel are prevented from being scalded.
Owner:COMAC +1
Esomeprazole magnesium enteric capsules and preparation method thereof
ActiveCN105106168AAvoid damageIntegrity guaranteedOrganic active ingredientsDigestive systemActive agentPharmaceutical drug
The invention discloses esomeprazole magnesium enteric capsules and a preparation method thereof. Esomeprazole magnesium is prepared into enteric coated pellets, and the enteric coated pellets are filled into gastric coated capsules. Each enteric coated pellet consists of an empty pellet core, a medicine carrying layer, two isolating layers, an enteric coated layer and a film coating layer. In order to guarantee stability of medicines, alkaline materials are added in the empty pellet cores; alkali stabilizers and antioxidant are added in the medicine carrying layer; and a high-alkalinity modifier and a low-alkalinity modifier are respectively added in the two isolating layers of each enteric coated pellet. In order to guarantee a dissolution effect, surfactant is added in the enteric coated layers; and stability of products is improved owing to the film coating layers wrapping the outer layers of the enteric coated pellets. Owing to optimized formulation and technology of the pellets, smoothness of a technology is improved, work efficiency is also improved, coating time is shortened, consumption of labor and materials is reduced, and production cost is reduced.
Owner:DEZHOU DEYAO PHARMA
Coated sherical seamless filled capsules
Described is a spherical coated capsule comprising (a) a coating-free capsule having (i) a liquid or viscous core and (ii) a seamless solid shell surrounding this core, and (b) a seamless, solid coating surrounding said coating-free capsule, wherein - the diameter of the coated capsule is in the range of 5 - 9 mm, - the solid coating comprises at least one sugar or sugar-alcohol in an amount from about 30 - 90% (m / m), based on the total mass of the coated capsule, - the diameter of the coating-free capsule is in the range of 3 - 7 mm, the thickness of the shell of said coating-free capsule is in the range of 20 - 200 m, - the ratio of shell thickness to diameter of said coating-free capsule is in the range of 0.004 - 0.04 , - the shell of said coating-free capsule contains 70 - 90 % (m / m) gelatine or alginate and 10 - 30 % (m / m) plasticiser, based on the solids content of said shell, and - the core has a flavouring content in the range of 1 - 100 % (m / m), based on the total mass of the core. Further described is a method for preparing such capsule.
Owner:SYMRISE GMBH & CO KG
Extended release film-coated capsules
Pharmaceutical formulations, preferably in the form of softgel capsules or hard-shell capsules, exhibit extended release through the use of a coating comprising a water-insoluble polymer and a pH-independent pore former. Extended release from softgel capsules and hard-shell capsules can be achieved without the use of lipid-based semi-solid or solid materials.
Owner:R P SCHERER TECH INC
Lansoprazole compound and pharmaceutical composition thereof
ActiveCN103254174AImprove stabilityLow impurity contentAntibacterial agentsOrganic active ingredientsLansoprazoleCoated tablets
The invention relates to a lansoprazole compound and a pharmaceutical composition thereof. The lansoprazole compound is a crystal. The characteristic peaks measured by X-ray powder diffraction are shown as 3.6, 4.8, 10.9, 14.0, 15.4, 16.9, 22.6, 24.7, 28.5, 32.1, 35.2, 36.7 and 39.3 at 2theta+ / -0.2 degrees. The medical composition preparation comprises the lansoprazole compound. The preparation is in form of freeze-dried powder injections, troches, capsules, enteric-coated tablets or enteric-coated capsules. The lansoprazole compound prepared by the invention has better solubleness and stability. The lansoprazole pharmaceutical composition prepared by using the lansoprazole compound provided by the invention as the effective component is good in stability.
Owner:湖北美林药业有限公司
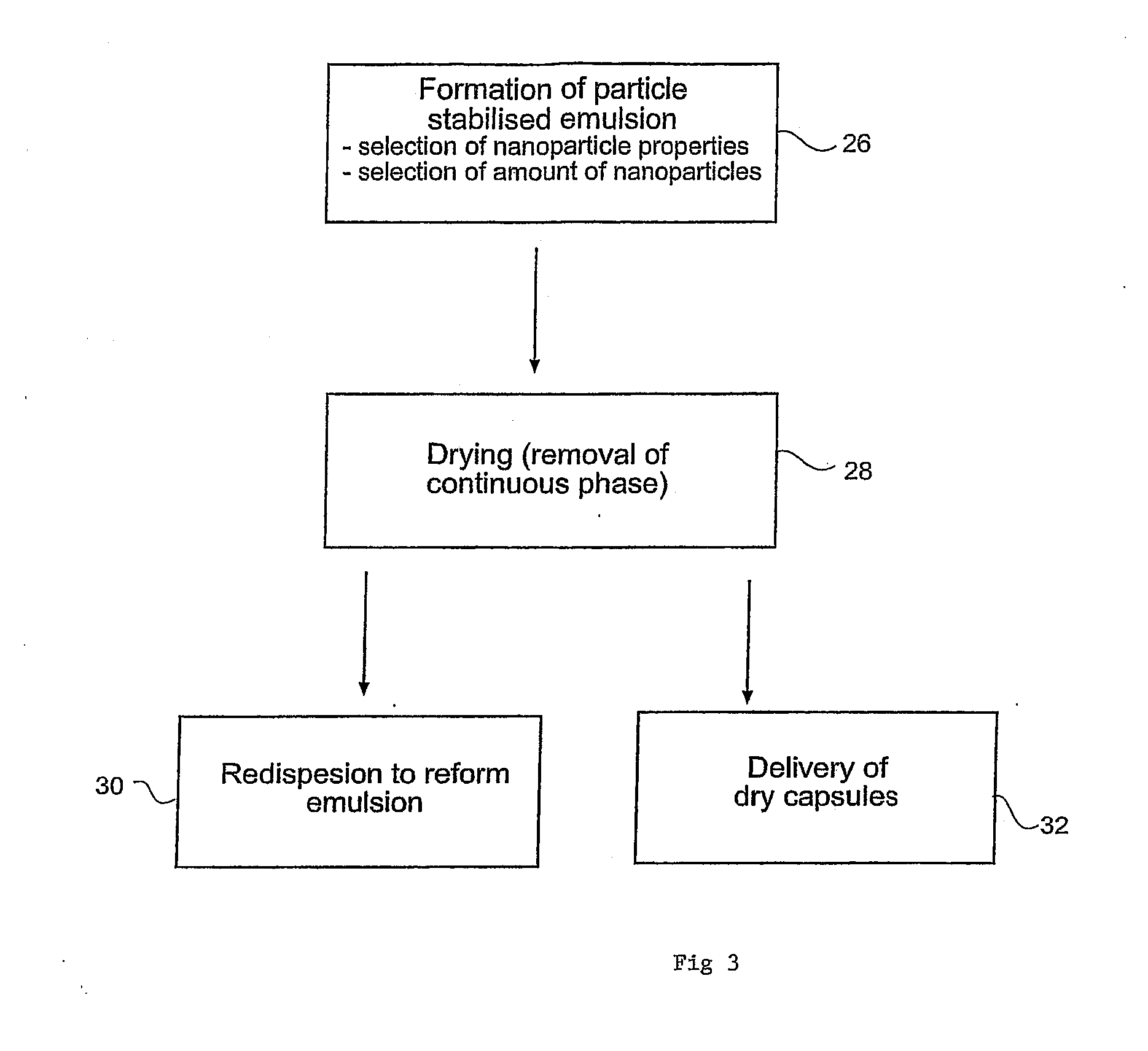
Dried Formulations of Nanoparticle-Coated Capsules
InactiveUS20080193513A1Improve bioavailabilityImprove adhesionPowder deliveryCosmetic preparationsPrillDrug compound
A method of producing a dried formulation for an active substance such as a drug compound is described. The method involves dispersing a discontinuous phase (e.g. an oil-based or lipidic medium) comprising the active substance into a continuous phase (e.g. water) so as to form a two-phase liquid system comprising droplets of said discontinuous phase, allowing nanoparticles to congregate at the phase interface at the surface of the droplets such that at least one layer of nanoparticles coat the droplets and thereby provide sufficient structural integrity to the droplets to enable the subsequent removal of the continuous phase, and thereafter removing the continuous phase from the nanoparticle-coated droplets to produce a dried formulation.
Owner:UNIVERSITY OF SOUTH AUSTRALIA
Aspirin enteric-coated pellet
ActiveCN101596166BEasy accessAvoid stimulationOrganic active ingredientsAntipyreticAspirinBlood concentration
The invention discloses an aspirin enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 5-22 blank core pellet, 55-75 medicinal layer consisting of aspirin and medicinal excipients and 20-35 enteric-coated layer. The invention also discloses a method for preparing the aspirin enteric-coated pellet and an aspirin enteric-coated capsule containing the aspirin enteric-coated pellets. The aspirin enteric-coated capsule has the advantages of good stability, small stimulation, steady blood concentration, high bioavailability, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Traditional Chinese medicine capsule capable of clearing heat and removing toxicity
InactiveCN103393992ASignificant effectQuick resultsAntinoxious agentsCapsule deliverySide effectWarm water
The invention provides a traditional Chinese medicine capsule capable of clearing heat and removing toxicity. The traditional Chinese medicine capsule comprises following raw materials, by weight: 10 to 15 portions of lonicera japonica, 5 to 10 portions of folium isatidis, 10 to 20 portions of fructus forsythia, 10 to 20 portions of coptis chinensis, 5 to 10 portions of radix scutellariae, 10 to 30 portions of radix isatidis, 10 to 20 portions of burdock seed, 10 to 20 portions of rhizoma phragmitis, 5 to 10 portions of bamboo leaf, 10 to 20 portions of rhizoma smilacis glabrae and 5 to 10 portions of gardenia jasminoides ellis. The traditional Chinese medicine capsule is prepared by following steps: taking the raw materials at the weight ratio, grinding the raw material into powder and adding the mixture into a sugar-coated capsule. The traditional Chinese medicine capsule is taken with warm water. Advantages of the traditional Chinese medicine capsule are that: curative effect is substantial; effectiveness is fast; the traditional Chinese medicine capsule is capable of clearing heat and removing toxicity, and possesses no side effects; and cost is low.
Owner:孟钧
Pantoprazole sodium compound and pharmaceutical composition thereof
ActiveCN103012373APowder deliveryOrganic active ingredientsPharmaceutical SubstancesOrganic chemistry
The invention relates to a pantoprazole sodium compound which is a crystal and is determined by X-ray powder diffraction. Characteristic peaks of the pantoprazole sodium compound are shown to be 7.2, 9.1, 11.0, 11.8, 14.7, 16.1, 18.4, 21.0, 23.5 and 25.2 at 2thet in a map. The invention also relates to a preparation method and a pharmaceutical composition of the pantoprazole sodium compound. The pharmaceutical composition comprises injection powder injection, enteric-coated tablet or enteric-coated capsule. Compared with the prior, the prepared pantoprazole sodium compound and the pharmaceutical composition thereof have obvious advantage in terms of quality stability.
Owner:湖北美林药业有限公司
Enteric-coated capsule material composition
ActiveCN103977412AImprove machinabilitySmall water absorptionCapsule deliveryMacromolecular non-active ingredientsPolymer scienceGelatin
The invention provides a novel enteric-coated capsule material composition, which is concretely composed of a starch-gelatin-chitosan crosslinking compound, an enteric material, a softening agent, a pH conditioning agent, water and a coloring agent, and water can be directly added in the composition for preparing a glue solution or sheet of the enteric-coated capsules.
Owner:HUNAN ER KANG PHARMA
Coated capsules and methods of making and using the same
InactiveUS20070014847A1Good flexibilityReduce leakagePretreated surfacesCapsule deliveryPliabilityBiomedical engineering
The present invention relates to coated capsules with increased capsule shell pliability and resilience and methods of making the coated capsules. The present invention relates to methods of making coated capsules, the methods comprising applying a coating solution comprising sodium carboxymethylcellulose or sodium alginate to the exterior of a capsule to form a coating and drying the coating to produce a coated capsule.
Owner:DURAMED PHARMA
Preparation of colon-targeted preparation
ActiveCN101209247AMaintain colorMaintain transparencyDigestive systemPharmaceutical non-active ingredientsHard CapsuleMedicine
The invention relates to a preparation method of a colon targeting preparation, which adopts an ordinary capsule, the colon delivered drug is filled in the capsule, the sealing material is used for sealing at the sleeving position of a capsule shell, so as to prepare a liquid hard capsule, and the coating liquid of colon enteric coating is used for coating of the finished liquid hard capsule; the coating liquid of colon enteric coating is prepared by coating material, coating solvent and plasticizer by mixing; the fillers in the capsule can be used as the drug for the treatment of ulcerative colitis or colon cancer. The invention has the advantages that: the invention adopts the pH-sensitive coating material to carry out the coating for the liquid hard capsule, so as to achieve the purpose of colon targeting release; the invention adopts the ordinary gel capsule shell; compared with the prior art of selecting the special colonic-coated capsule shell, the machine using ratio is high, the coating process is stable, the operation is easy, the invention is applicable to the large-scale industrial production; the liquid hard capsule which is prepared by the method can maintain the luster and transparency of the original capsule after the coating, thus improving the patient compliance; the technique is simple, the special equipments are not needed, the operation is easy and the cost is low.
Owner:TIANJIN ZHONGXIN PHARMA GRP CO LTD
Enteric delivery of (-)-hydroxycitric acid
The present invention provides stable encapsulated (−)-hydroxycitric acid (“HCA”) dosage unit forms, uses thereof, as well as and methods of making the same. In particular, HCA and the salts, esters and amides of HCA according to the invention are delivered via enteric vehicles, such as enteric-coated tablets, and also enteric and enteric-coated capsules and soft gelatin capsules (softgels). Enteric-coatings may be applied externally to the HCA-containing dosage unit form or, in the case of capsules and soft gelatin capsules, the enteric compound also may be incorporated into the gelatin shell to yield an HCA-containing dosage unit form of the invention. The HCA-containing compositions are protected against acid degradation, lactonization and undesirable ligand binding in select environments. The invention provides HCA-containing dosage unit forms useful to prevent or reduce the symptoms associated with a disease, disorder or condition such as obesity, weight gain, hunger, hyperlipemia, and postprandial lipemia.
Owner:GLYKON TECH GRP
Enteric-coated capsule of lentinan and preparation method thereof
The invention discloses preparing method for mushroom polysaccharide capsule, comprising the following steps: using mushroom as raw material, soaking with water, milling, separating solid and liquid, carrying out ultra filtration, vacuum distilling, thickening, dissolving them with 95% ethanol, separating upper layer, getting precipitate, vacuum distilling to remove water, vacuum drying, extracting particle core, covering particle core with hydroxypropylcellulose, II acrylic resin and III acrylic resin, and getting the product.
Owner:苏少宁
Modified release coated capsules
ActiveUS20170079927A1Improve adhesionReduce the amount requiredOrganic active ingredientsCapsule deliveryPharmacologyCoated capsule
The present invention relates to a modified release coated capsule and a process to obtain that capsule.
Owner:TILLOTS PHARMA AG
Portable Cleaning Article and the Forming method thereof
ActiveUS20100323947A1Lower the volumeConvenient for userCosmetic preparationsHair cosmeticsCleansing AgentsBiomedical engineering
A portable cleaning article includes a coated capsule and cleaning agent, in which the cleaning agent is contained within the coated capsule, the characteristic in that: the coated capsule having a thin-film layer and an oil layer that is coated over the surface of the thin-film layer to form a portable cleaning article. When the coated capsule is brought into contact with water by the user, the coated capsule dissolves in water for the cleaning agent to exert its cleaning function.
Owner:SES MILD INT
Ramatroban glue pill and its preparation method
The invention discloses a Ramatroban gelatin-coated capsule pill comprising liquid solution with active constituent and outer wrapping material, wherein the liquid solution with active constituent is Ramatroban solution prepared from Ramatroban and findings, the invention also discloses the process for preparing the Ramatroban capsule and pill.
Owner:杭州容立医药科技有限公司
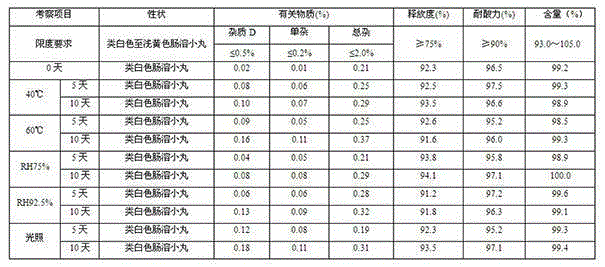
Oxiracetam enteric-coated preparation and preparation method thereof
InactiveCN109718222ALow dissolution rateGood storage stabilityOrganic active ingredientsNervous disorderEnteric-coated granulesDissolution
The invention provides an oxiracetam enteric-coated capsule. Oxiracetam in special crystallization form is used as an active component. A composite carrier containing polyoxyethylene PEO and hydroxypropyl methyl cellulose ( or polyvinylpyrrolidone) in the mass ratio of the polyoxyethylene PEO to the hydroxypropyl methyl cellulose being (1-3) to 1, is carefully selected, and the prepared oxiracetamenteric-coated preparation can reduce the dissolution rate of the active component in the stomach, and also has favorable slow control slow release properties and storage stability. The prepared oxiracetam enteric-coated granules are subjected to long-term stability test under the condition that the temperature is 25 DEG C+ / -2 DEG C, and the relative humidity is 60%+ / -10%, and after long-term experiment for 24 months, sample properties, content and related substances all conform to specification. The oxiracetam enteric-coated capsule is good in stability, and long in quality guarantee period.The preparation method is simple, and suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
Phosphorus-free, fluorescent agent-free and preservative-free super-concentrated capsule granule laundry detergent, preparation method and use method thereof
InactiveCN106566732ASurface-active detergent compositionsSurface-active non-soap compounds and soap mixture detergentsActive agentFluorescence
The invention discloses a phosphorus-free, fluorescent agent-free and preservative-free super-concentrated capsule granule laundry detergent, a preparation method and a use method thereof, wherein the phosphorus-free, fluorescent agent-free and preservative-free super-concentrated capsule granule laundry detergent comprises 40-50% of fatty acid methyl ester sulfonate MES, 30-40% of natural soap powder, 1-5% of sodium olefin sulfonate AOS, 5-10% of sodium gluconate, 1-5% of isomeric alkanol ether EH-7, 1-3% of microcapsule essence, 1-2% of a suspending agent, 8-15% of coating powder, and 1-3% of granule compound enzyme. According to the present invention, the coating capsule concentration granulation process is used, the phosphorus-free, fluorescent agent-free and preservative-free super-concentrated capsule granule laundry detergent does not contain phosphorus, the fluorescent agent and the preservative, and the natural interface surfactant and the microcapsule essence are selected; and during the use, the skin irritation and the environmental pollution of the fluorescent agent, the preservative and the interface surfactant can be effectively reduced, and the aroma can emit during the washing process and can stay on clothing for a long time.
Owner:FENGHUA YAOQIN BIOTECH CO LTD
Modified release coated capsules
ActiveUS11266605B2Improve adhesionReduce the amount requiredCapsule deliveryBiomedical engineeringNanotechnology
The present invention relates to a modified release coated capsule and a process to obtain that capsule.
Owner:TILLOTS PHARMA AG
Enteric-coated capsule containing cationic nanoparticles for oral insulin delivery
ActiveUS9101547B2Promote absorptionPeptide/protein ingredientsMetabolism disorderMedicineNanoparticle
The invention relates to an enteric-coated capsule containing cationic nanoparticles for oral insulin delivery, in particular to a type of cationic nanoparticle including a polycationic and mucoadhesive polymer and a biodegradable polymer, wherein each of the nanoparticles has positive surface charge and enhanced permeability for paracellular insulin delivery; the enteric-coated capsule further includes a pH-sensitive polymer as the coating. The enteric-coated capsule containing cationic nanoparticles, when being orally administered to a subject, are configured to prevent the acidic degradation of the active substance such as insulin before being released from said cationic nanoparticles to a specific absorption site along the gastrointestinal tract.
Owner:NANO & ADVANCED MATERIALS INST
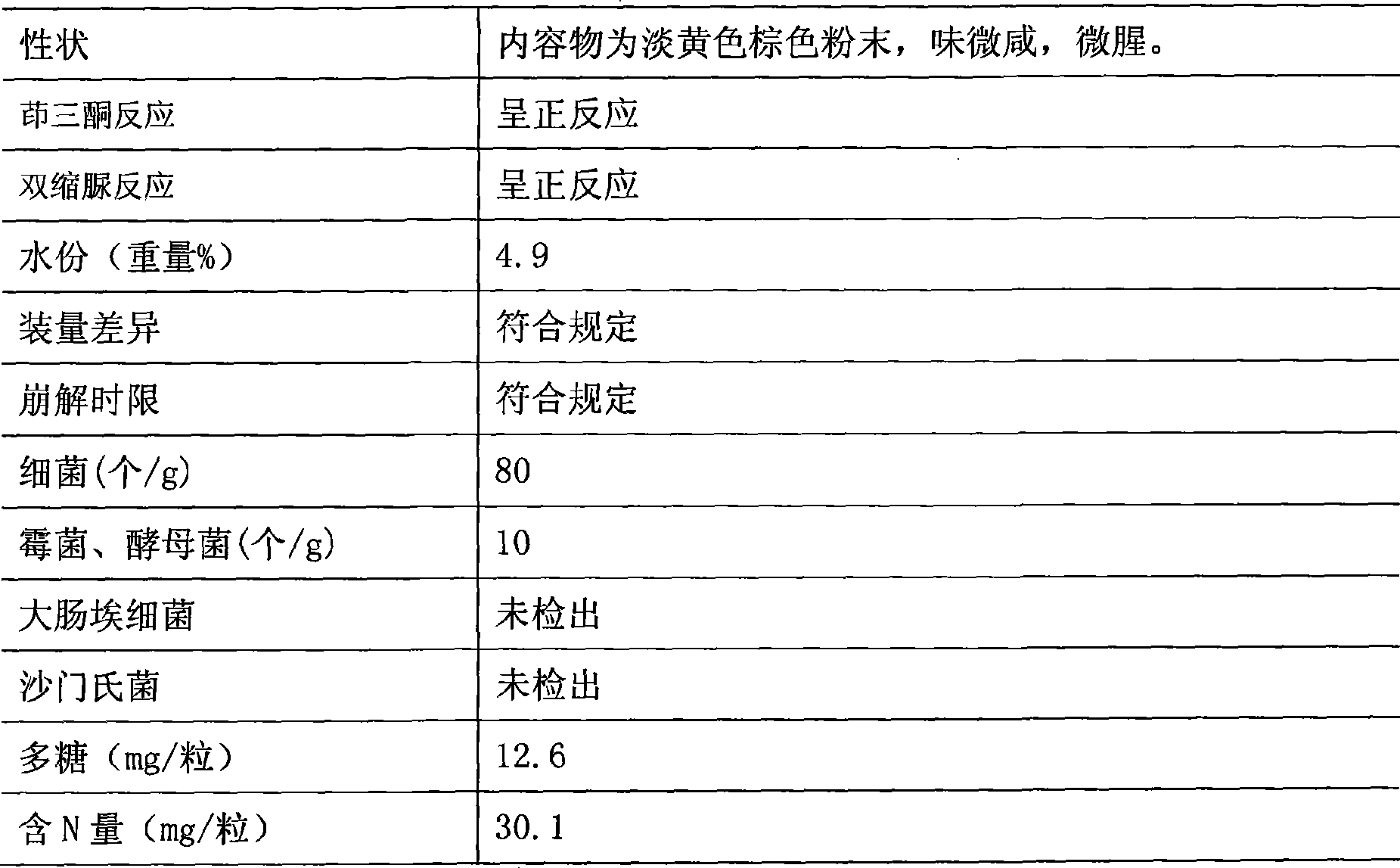
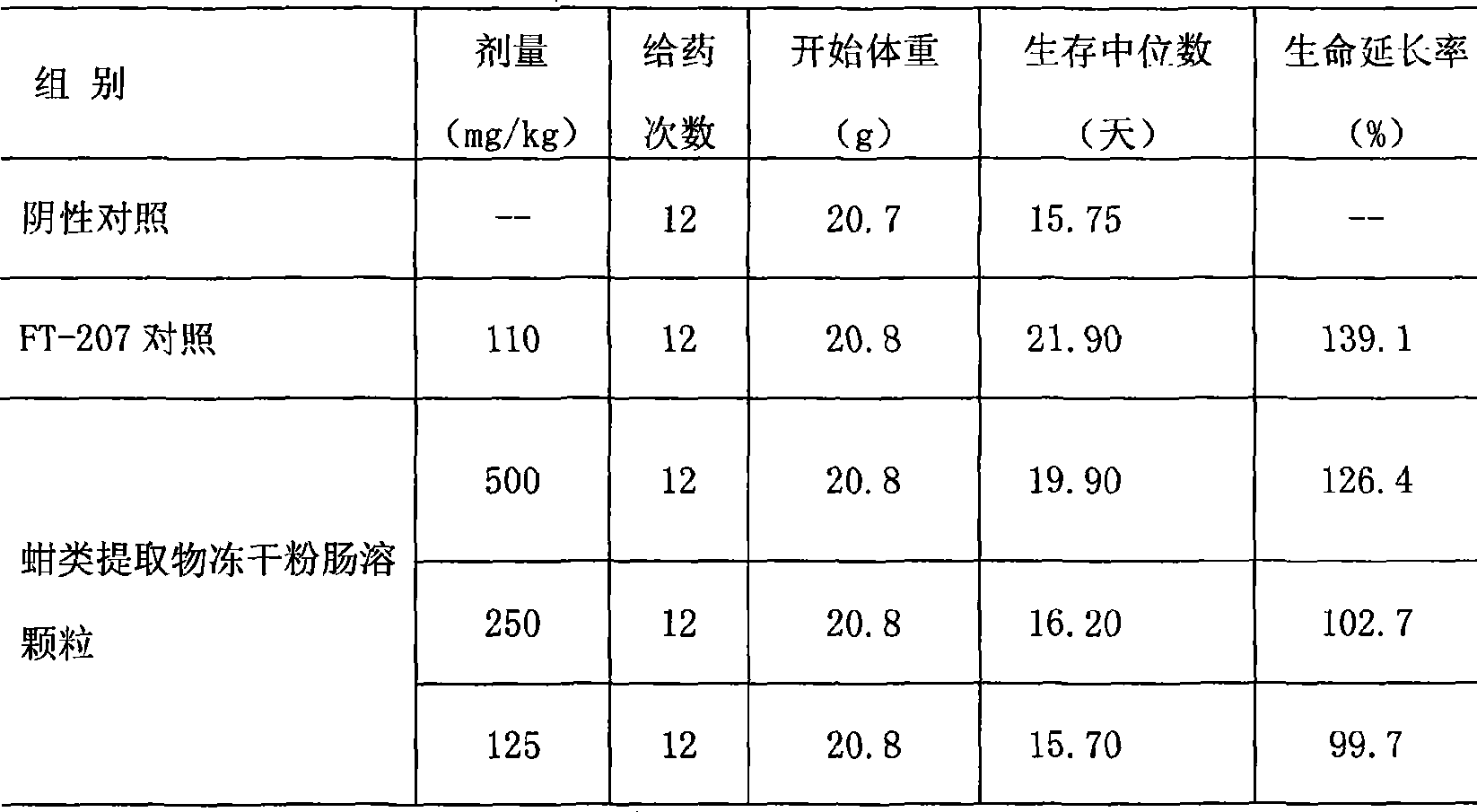
Arca extract enteric-coated capsules and manufacture method thereof
The invention relates to a clam extract enteric-coated capsule and a production method thereof. The clam extract enteric-coated capsule comprises a clam extract freeze-dried powder and an accessory, the weight of the accessory is 3 percent to 5 percent of the clam extract freeze-dried powder, wherein, the accessory is silicon dioxide and magnesium stearate, the weight ratio of which is 1:1 to 2:1. The clam extract freeze-dried powder is obtained by the steps of cleaning marine organism clams, removing shells, taking out soft bodies, mashing, extracting and freeze-drying. The clam extract enteric-coated capsule is rich in resources and easy to product, has high biological activity, can significantly improve the symptoms of malignancy, decreases myoma volume, enhances the quality of life, prolongs life cycle and can be applied to treating malignancy patients, such as lung cancer, liver cancer, renal carcinoma, carcinoma ventriculi, cervical carcinoma patients and the like and no obvious toxic and side effect is found.
Owner:青岛海生肿瘤医院
Capsule type slow-release formaldehyde scavenger and preparation process thereof
ActiveCN113181767AContinuous adsorption purificationSatisfy Absorption ClearanceMaterial granulation and coatingGas treatmentSustained Release CapsuleTitanium oxide
The invention discloses a capsule type slow-release formaldehyde scavenger and a preparation process thereof, and belongs to the technical field of formaldehyde scavenging. The capsule-type slow-release formaldehyde scavenger comprises a slow-release carrier pellet core and a thinly coated capsule. The capsule type slow-release formaldehyde scavenger is prepared from the following raw materials in parts by mass: 40-70 parts of an activated carbon base material, 30-60 parts of a titanium dioxide base material, 5-10 parts of a forming material, 2-20 parts of a sustained-release material, 1-5 parts of a plasticizer and 0.5-1.5 parts of an anti-sticking agent. The thinly coated capsule is a polytetrafluoroethylene breathable waterproof thin coating, and the preparation process comprises matrix preparation, material mixing, granulation, sustained-release liquid preparation and coating. The titanium dioxide oxide is mixed in the activated carbon matrix, and after the activated carbon, the forming material and the sustained-release material are fused, the sustained-release capsule can continuously adsorb and purify formaldehyde under photocatalysis, and a surface film can isolate external water vapor from soaking, so that the sustained-release efficiency is improved, the coating placement time is remarkably prolonged, and the continuous purification capability on formaldehyde is met.
Owner:JIANGSU OPEN UNIV
Method of producing penetrating pump capsule as one new dosage form
InactiveCN1370522AGood reproducibilityHigh feasibilityCapsule deliveryBiomedical engineeringCoated capsule
The present invention is method of producing penetrating pump capsule as one new dosage form from common capsule. This kind of new dosage has the features of both common gastric coated capsule and enteric coated capsule and its medicine is controlled to release safely and in long action and absorbed in both stomach and intestines in certain propertion. With material similar those in common capsule, the medicine and active penetrating supplementary material are filled into capsule, and the capsule coating is coated with coating liquid in certain thickness before one or several holes are drilled.
Owner:SHENYANG PHARMA UNIVERSITY
Injection molding method and device for preparing large-size high-temperature-resistant and high-pressure-resistant expansion coated capsule from ultrahigh-viscosity silicone rubber
The invention discloses an injection molding method and device for preparing a large-size high-temperature-resistant and high-pressure-resistant expansion coated capsule from ultrahigh-viscosity silicone rubber. Part of the composite material has a large-size complex internal structure, so that the composite material has difficulties in preparing and demoulding processes, and the problems of uneven mixing, poor storage and the like of the double-component high-viscosity rubber in a rubber injection process are extremely prone to occurring during injection molding. The invention provides the injection molding method and device for preparing the large-size high-temperature-resistant and high-pressure-resistant expansion coated capsule from the ultrahigh-viscosity silicone rubber in order tosolve the problems, and relates to a two-component resin tank, an extrusion device, a vacuum device, a mixer and a mold. A material A and a material B are independently stored in the material storagebox, a vacuum device is connected into the material storage box, the material A and the material B in a ratio of x:y can be extruded into the mixer through an extrusion device during use, the mixed material passes through the mutichannel mixer during injection into the mold, rotating blades are installed in the mixer, and finally the mixed material is injected into the mold. According to the invention, rubber products with certain thickness, temperature resistance, long size and anisotropy can be produced.
Owner:TIANJIN POLYTECHNIC UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com