Enteric-coated capsule material composition
A material composition, enteric technology, applied in the field of medicine, can solve the problems of increased process and cost, poor film-forming performance, expensive, etc., to improve stretchability and stability, overcome poor performance of mixed materials, save money Process and cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
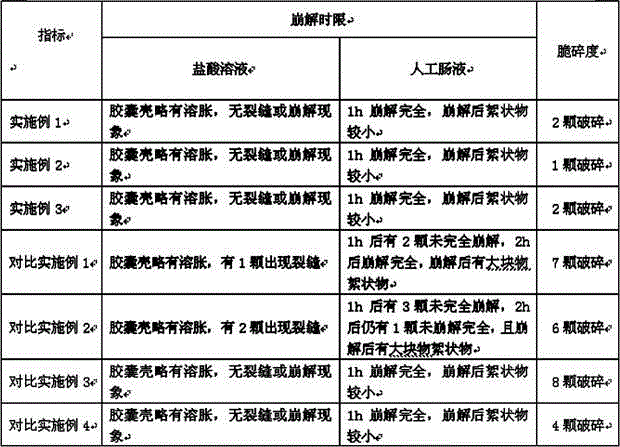
Embodiment 1
[0049] Get 70 parts of the starch-gelatin-chitosan cross-linked compound, add 5 parts of enteric acrylic resin, 10 parts of glycerin, 0.1 part of sodium hydroxide and 10 parts of water, mix well, and make wet granules through a 24-mesh sieve. The wet granules were dried in a vacuum oven at 50°C to obtain the capsule material composition. The composition is used to prepare hollow hard capsules by adopting the existing glue-dipping method.
Embodiment 2
[0051] Take 85 parts of starch-gelatin-chitosan cross-linked compound, add 2 parts of polyethylene acetate phthalate, 3 parts of polyvinyl alcohol, 3 parts of propylene glycol, 2 parts of polyethylene glycol 200, 0.1 part of sodium hydroxide and 10 parts of water, mixed evenly, passed through a 24-mesh sieve to make wet granules, and dried in a vacuum oven at 50°C to obtain the capsule material composition. The composition is used to prepare hollow hard capsules by adopting the existing glue-dipping method.
Embodiment 3
[0053] Take 75 parts of starch-gelatin-chitosan cross-linked complex, add 15 parts of hydroxypropylmethylcellulose acetate succinate, 5 parts of triethyl citrate, 0.5 parts of potassium hydroxide and 10 parts of water, mix well, Wet granules are made through a 24-mesh sieve, and the wet granules are dried in a vacuum oven at 50° C. to obtain the capsule material composition. The composition is used to prepare hollow hard capsules by adopting the existing glue-dipping method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com