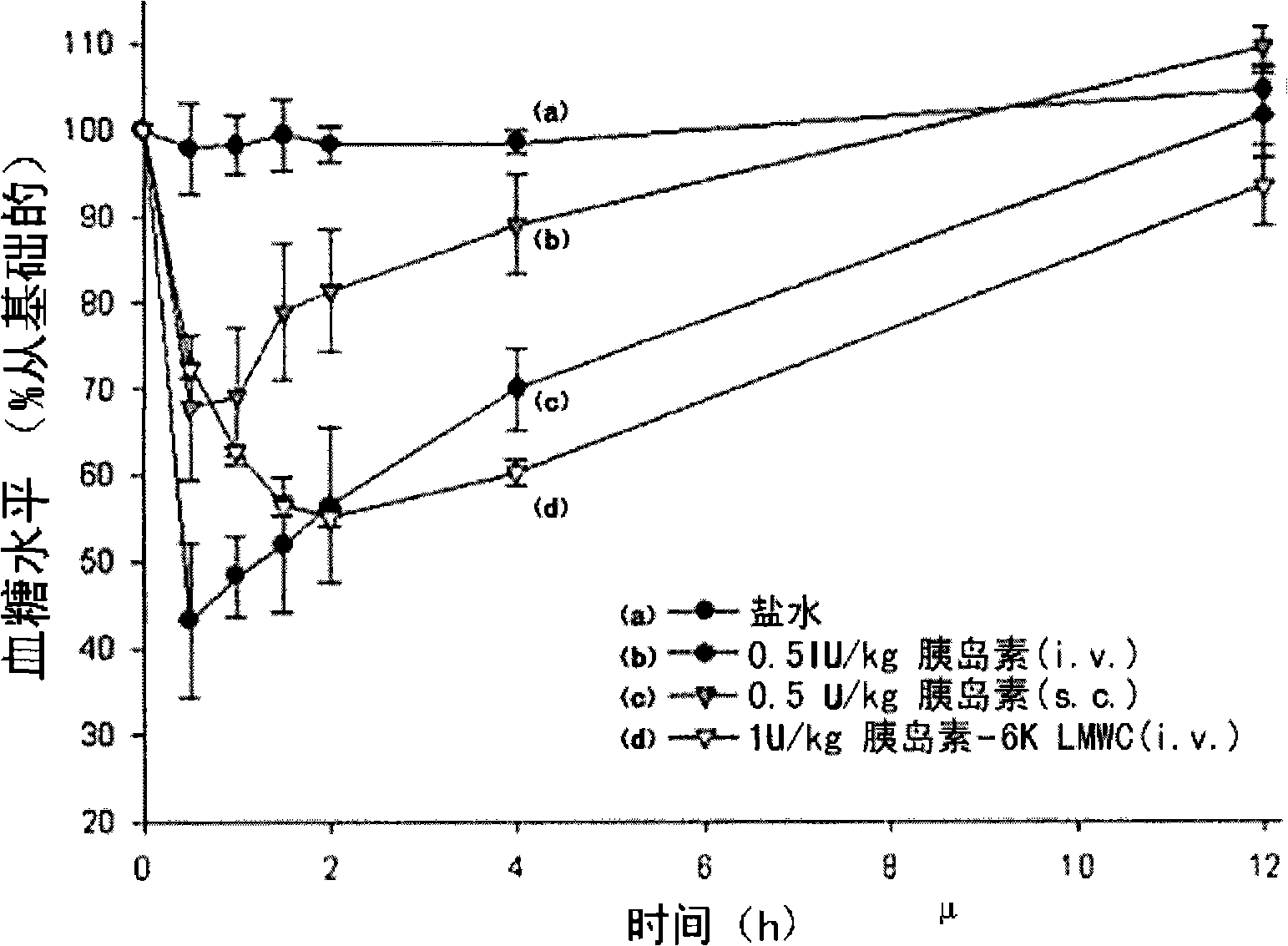
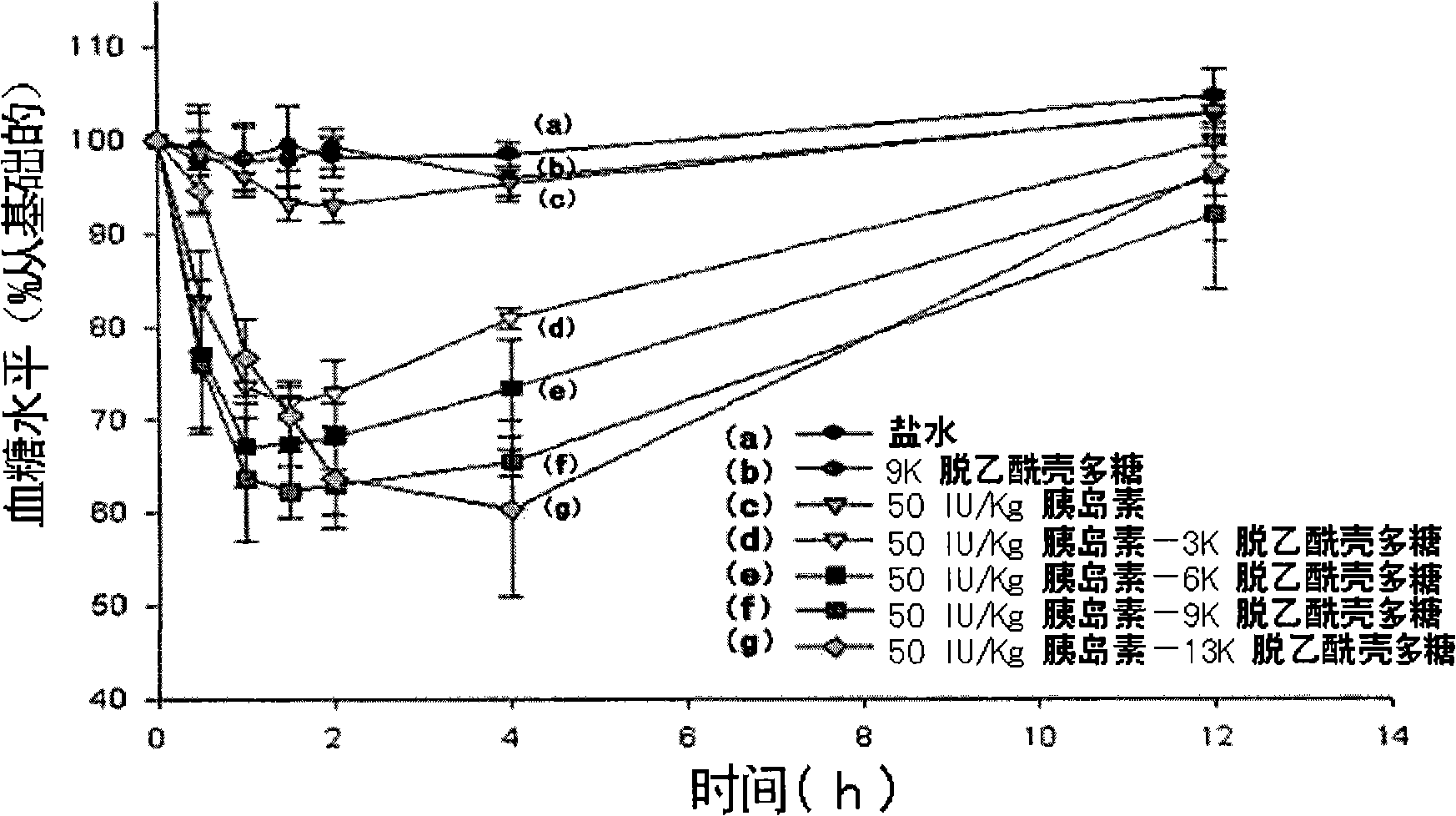
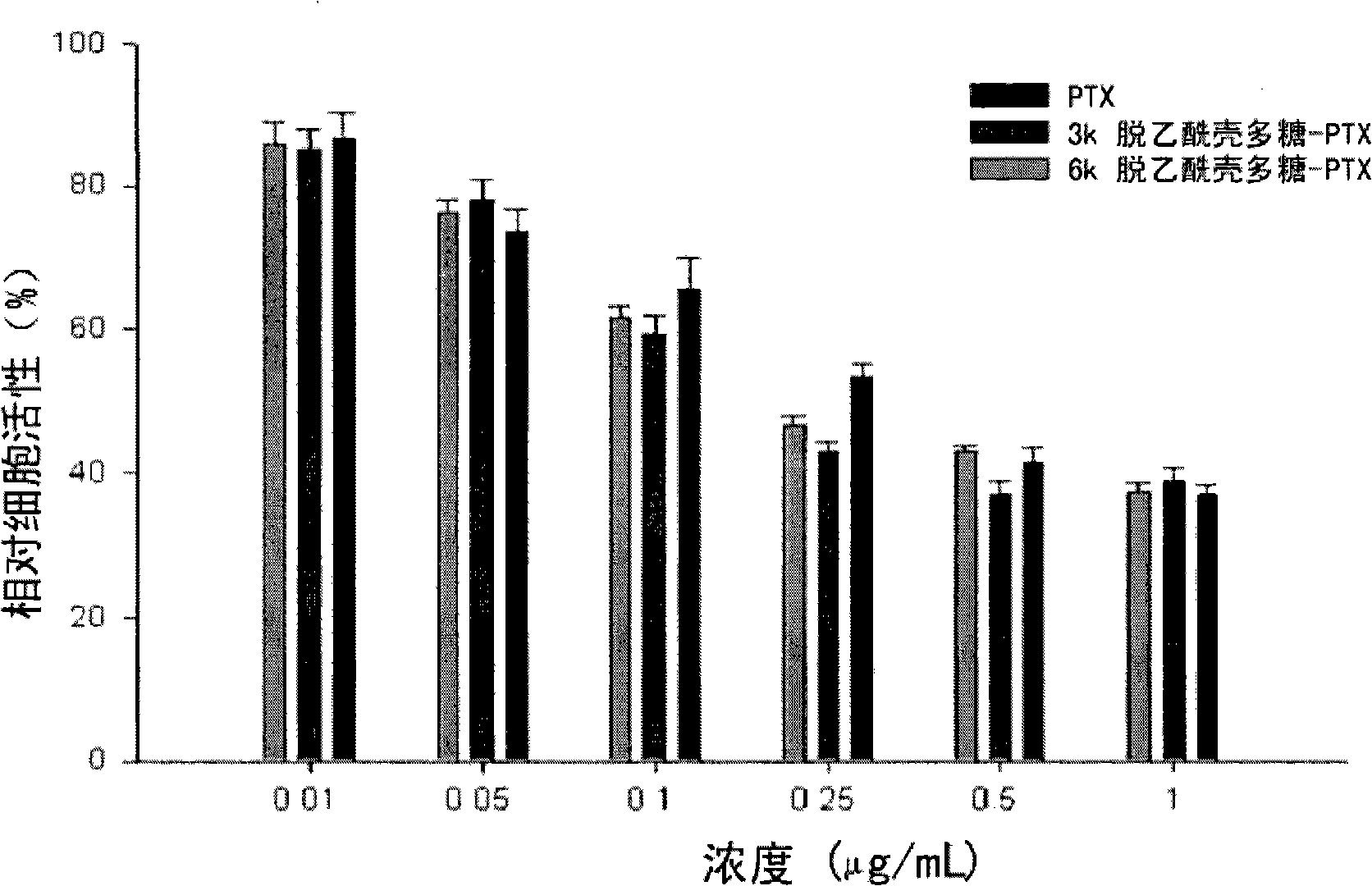
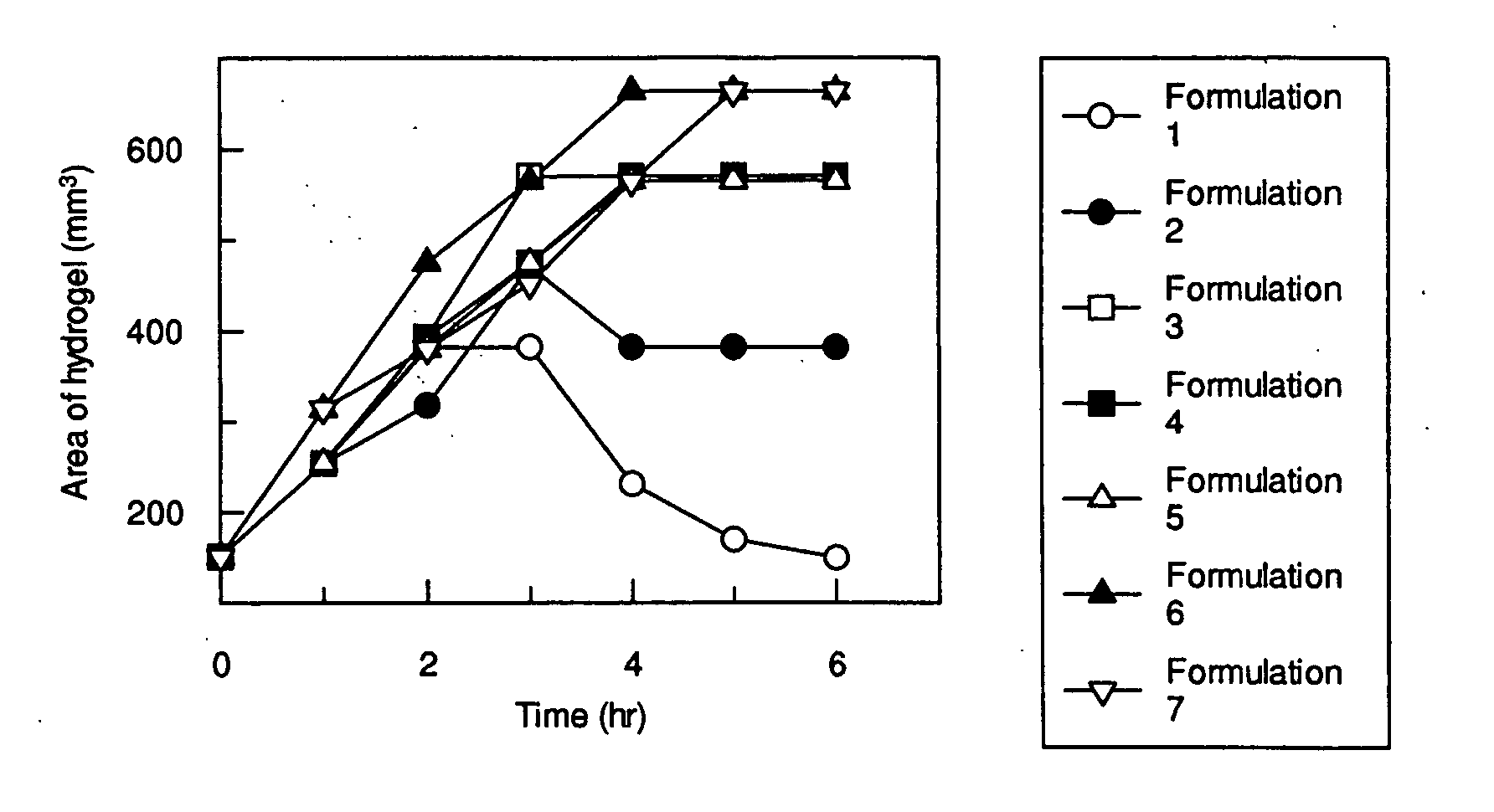
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Mucoadhesive polymers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral mucoadhesive dosage form
ActiveUS20110028431A1Improve stabilityPromote absorptionOrganic active ingredientsBiocideActive agentPharmacology
A direct compression formulation suitable for preparing buccal and / or sublingual and dosage forms incorporates a combination of a non-ionic polymeric solubility enhancer, a mucoadhesive polymer, a filler, a disintegrant, and a pharmaceutically active agent. Cannabinoid-cyclodextrin complexes exhibiting an improved property selected from improved stability, higher product yield and improved product uniformity may be obtained by complexing the cannabinoid with the cyclodextrin in a liquid medium containing an antioxidant. To enhance stability, product yield and / or product uniformity, complexing may be done while the liquid medium is in contact with an atmosphere having a very low oxygen content. The resulting complexes may be combined with decomplexing agents and / or dispersed in a matrix material comprised of a hydrogel-forming polymer to provide enhanced absorption of the cannabinoid through oral mucosa and reduced ingestion of the cannabinoid as compared with known commercially available cannabinoid-containing oral dosage forms.
Owner:INTELGENX CORP
Composition for enhancing absorption of a drug and method
InactiveUS20050244502A1Avoid stimulationDifficult to handlePowder deliveryDispersion deliveryNoseCytotoxicity
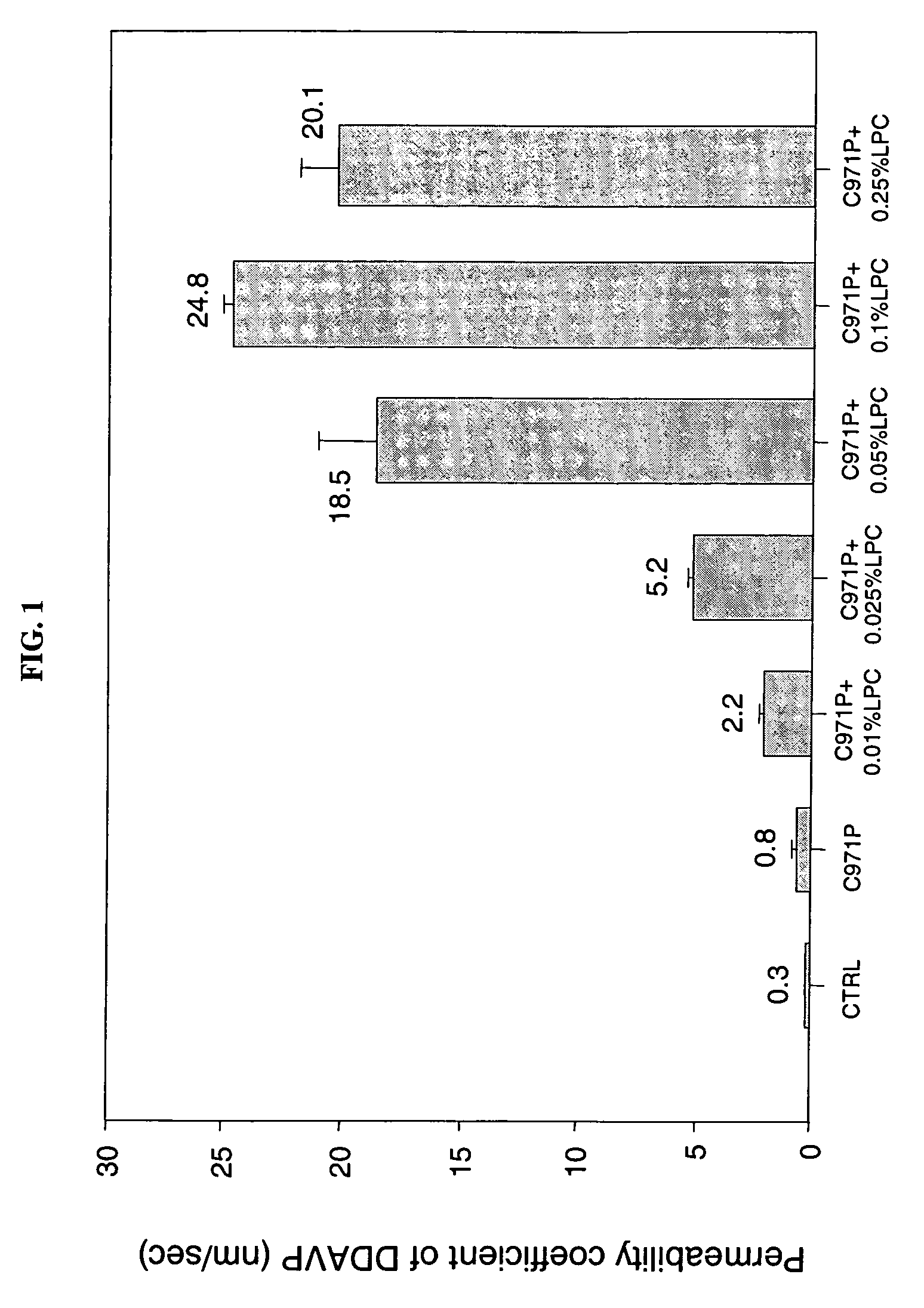
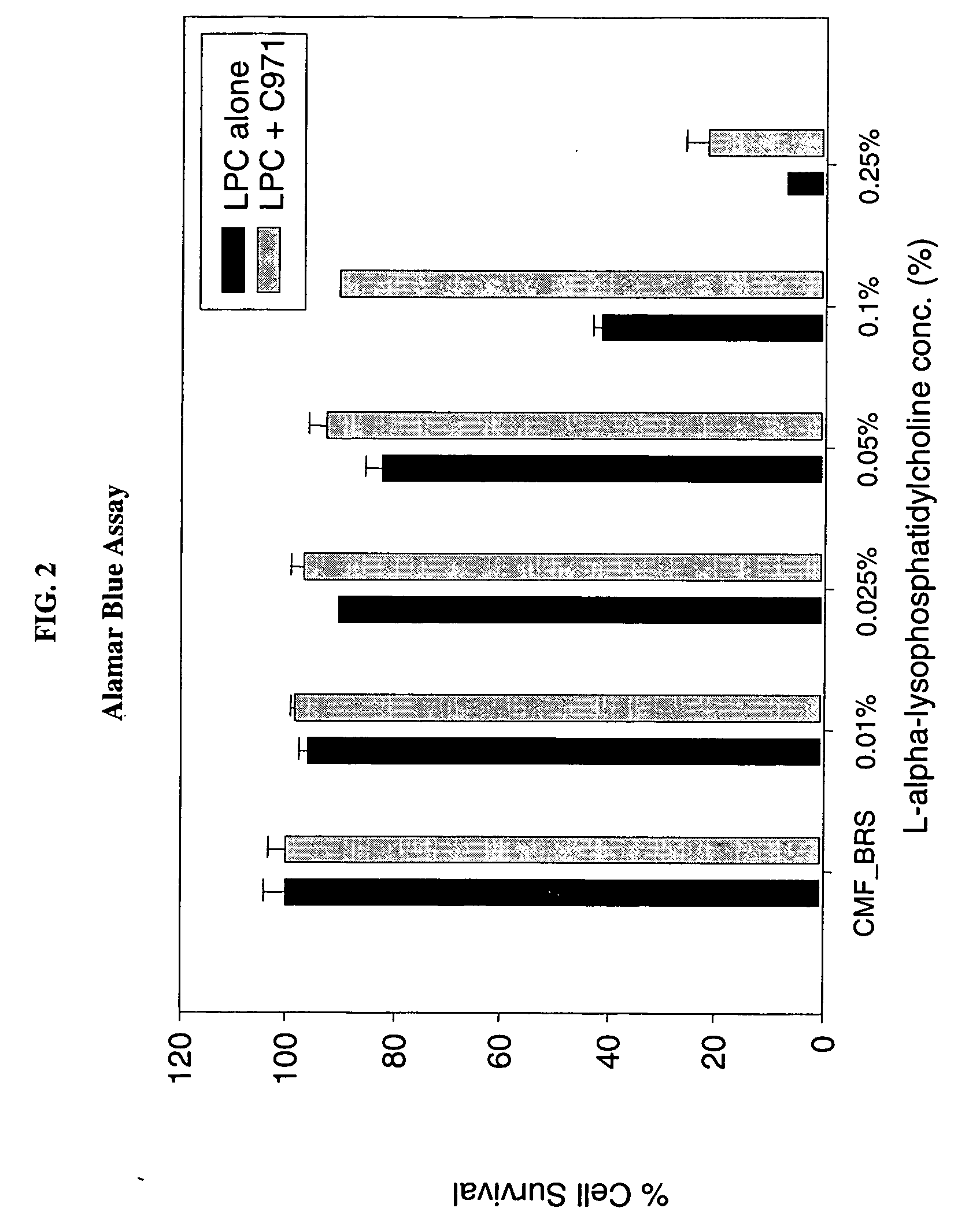
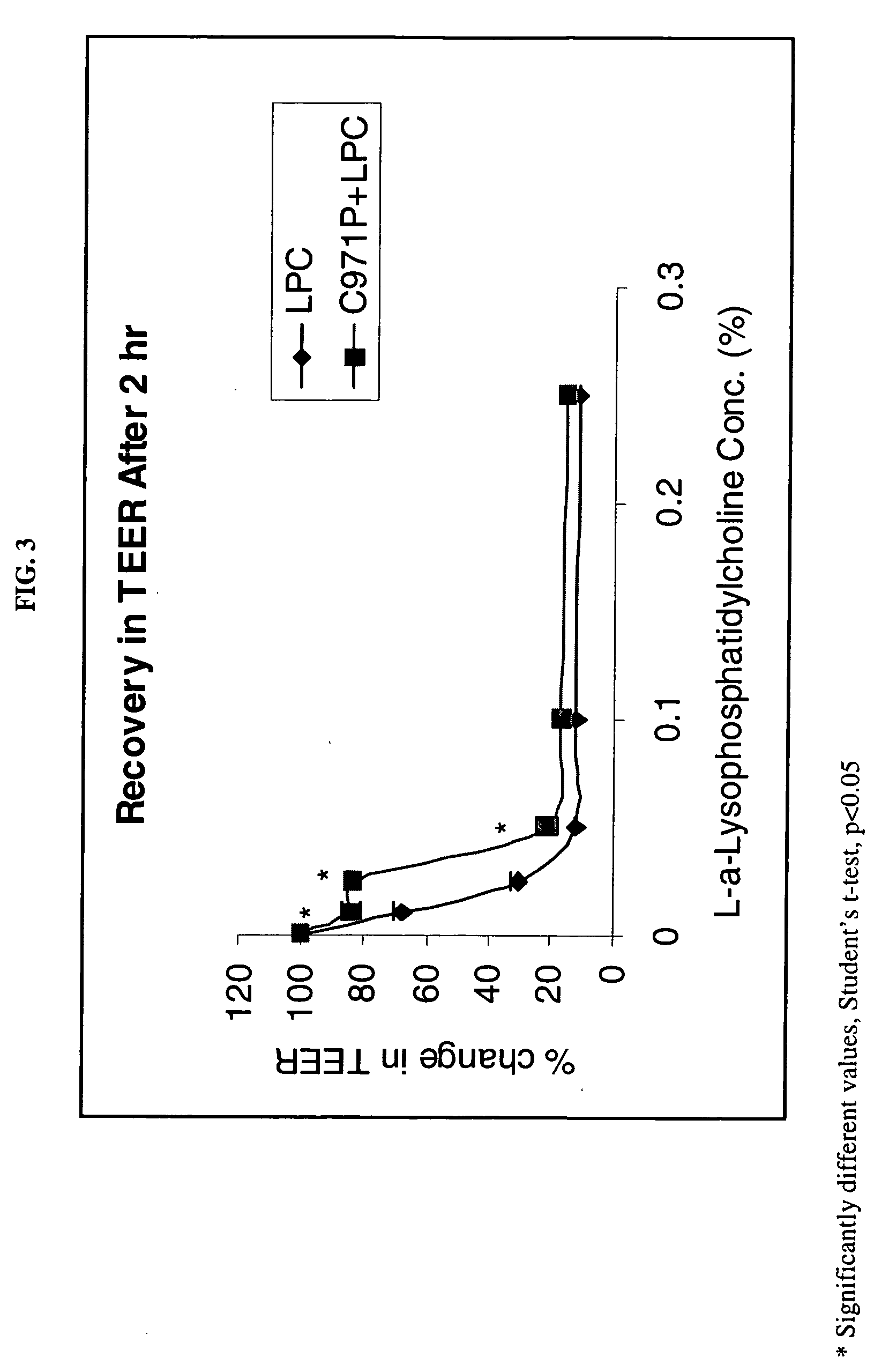
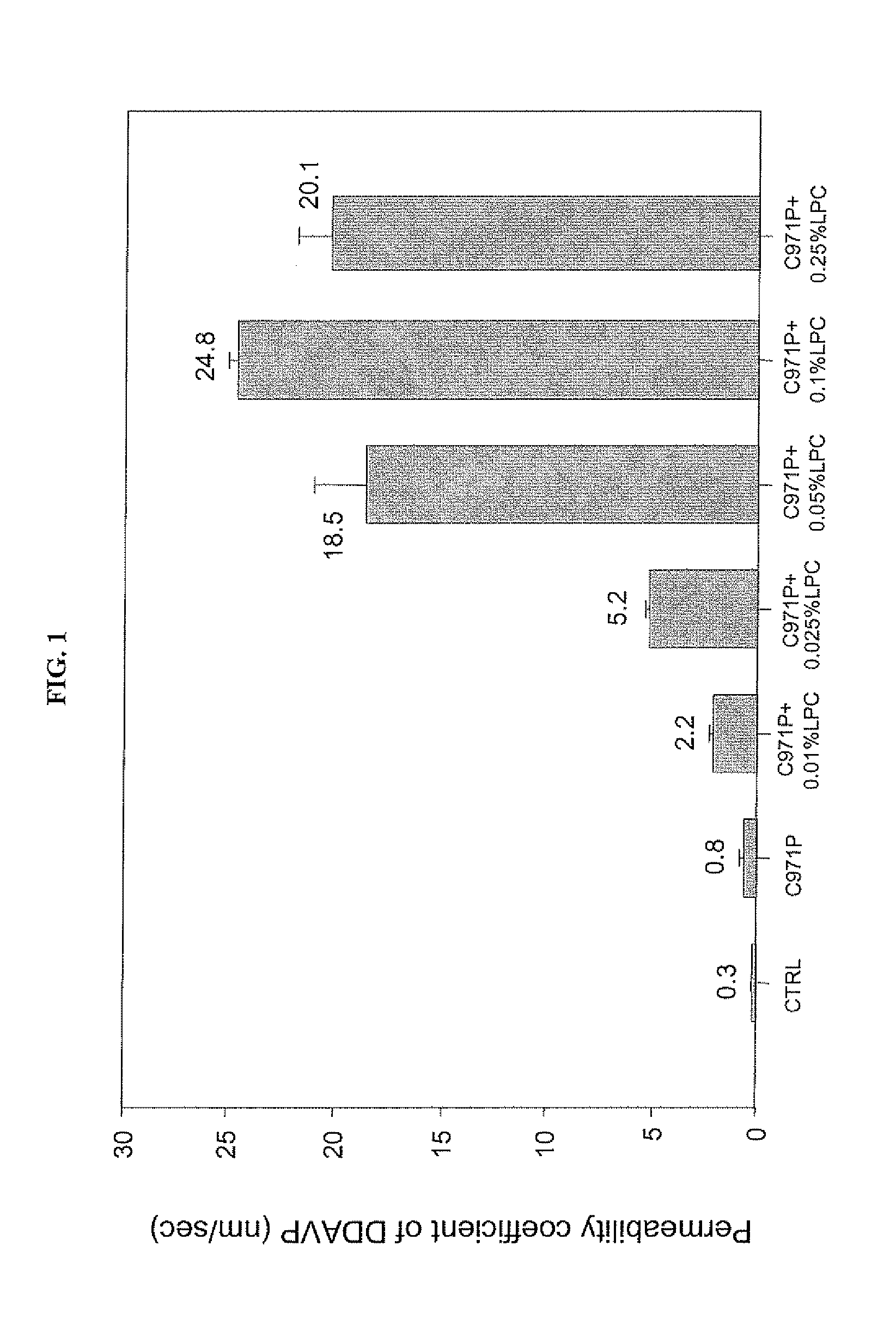
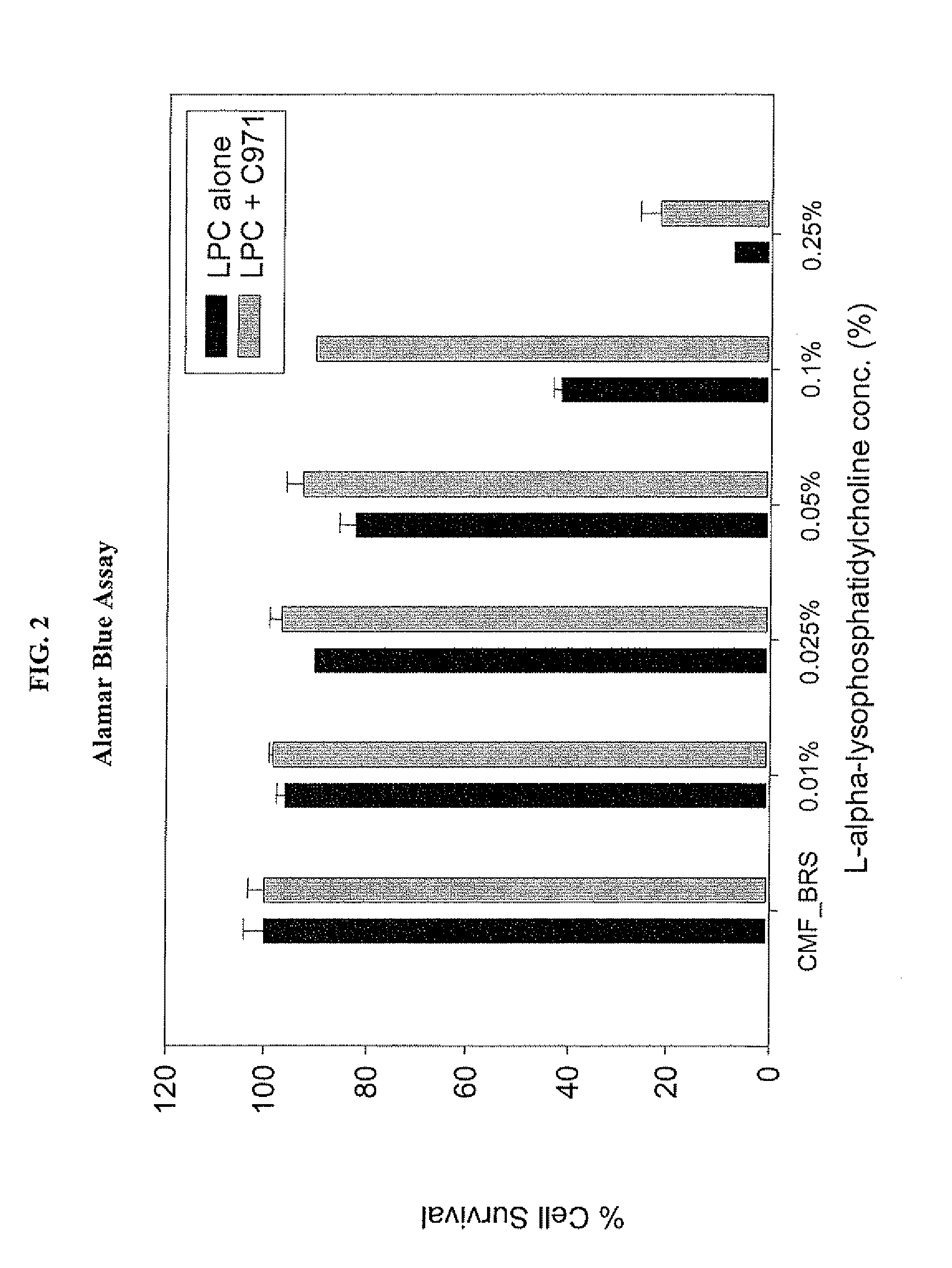
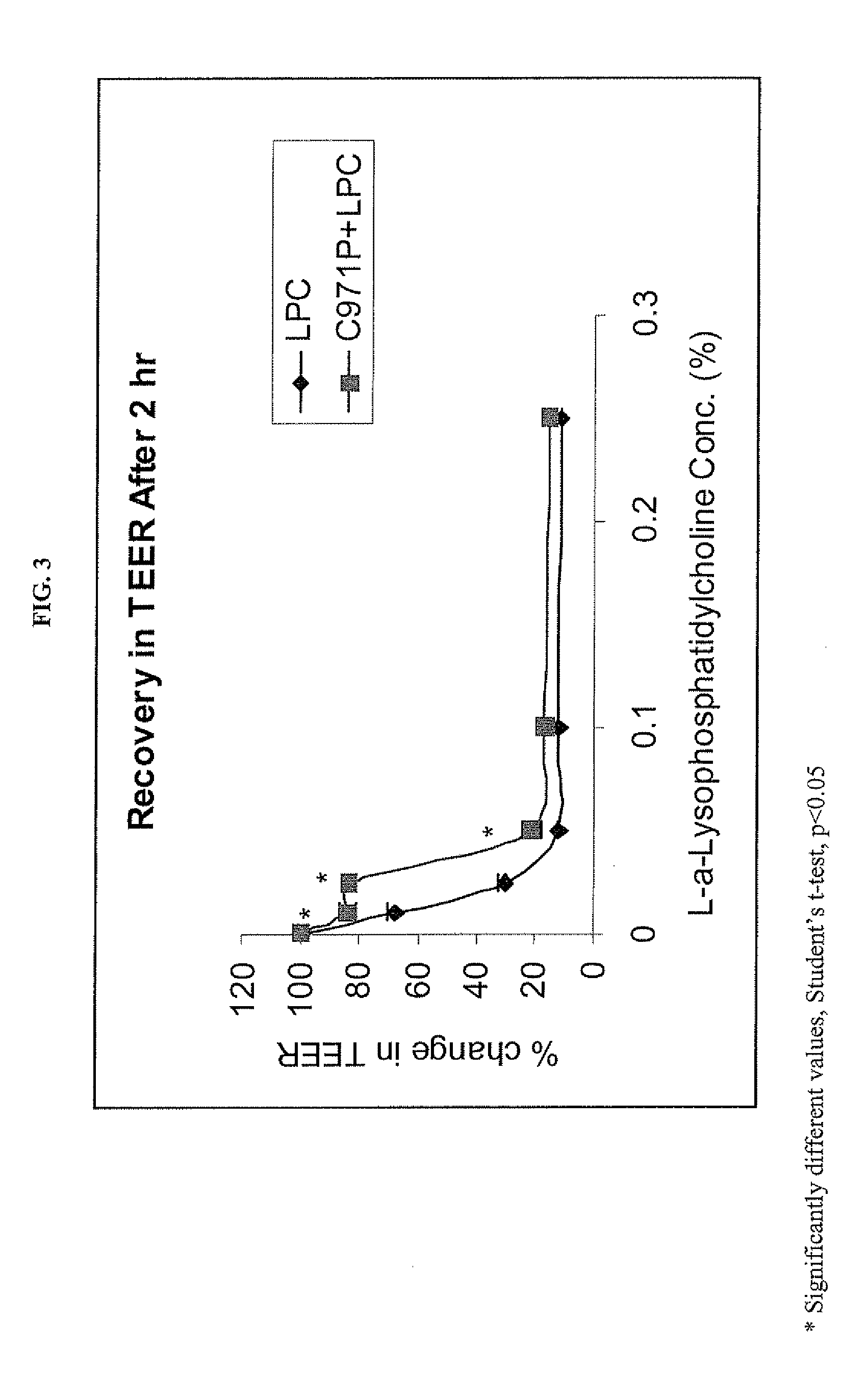
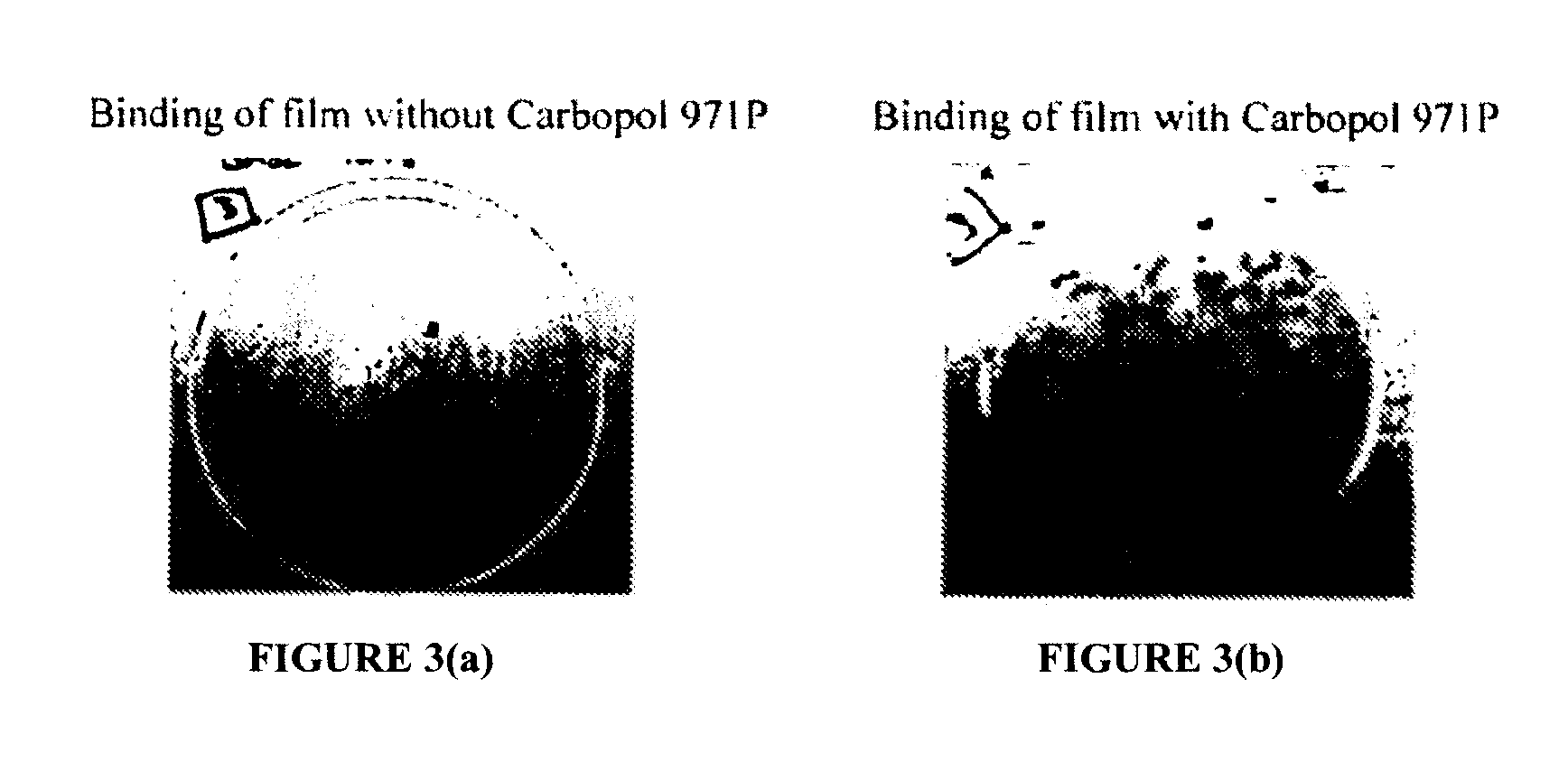
A composition for enhancing absorption of a pharmaceutical which may have poor oral bioavailability, which composition has surprisingly little cytotoxicity, is provided which is in the form of a liquid or semi-solid or solid containing an admixture (1) a mucoadhesive polymer which is a polyacrylic acid polymer, preferably Carbopol 971P, and (2) an absorption or permeation enhancer which preferably is L-α-lyso-phosphatidylcholine (LPC), and which composition is free of polysaccharides. A method for improving bioavailability of a drug which has poor absorption properties is also provided wherein the above bioadhesive composition is administered with said pharmaceutical to the mucosal membrane of the GI tract, nose, oral cavity, sublingual, buccal, and vaginal mucosa. A method for reducing the cytotoxic effect of an absorption enhancer such as LPC is also provided wherein a mucoadhesive polymer as described above is administered with the LPC to a patient in need of treatment.
Owner:BRISTOL MYERS SQUIBB CO
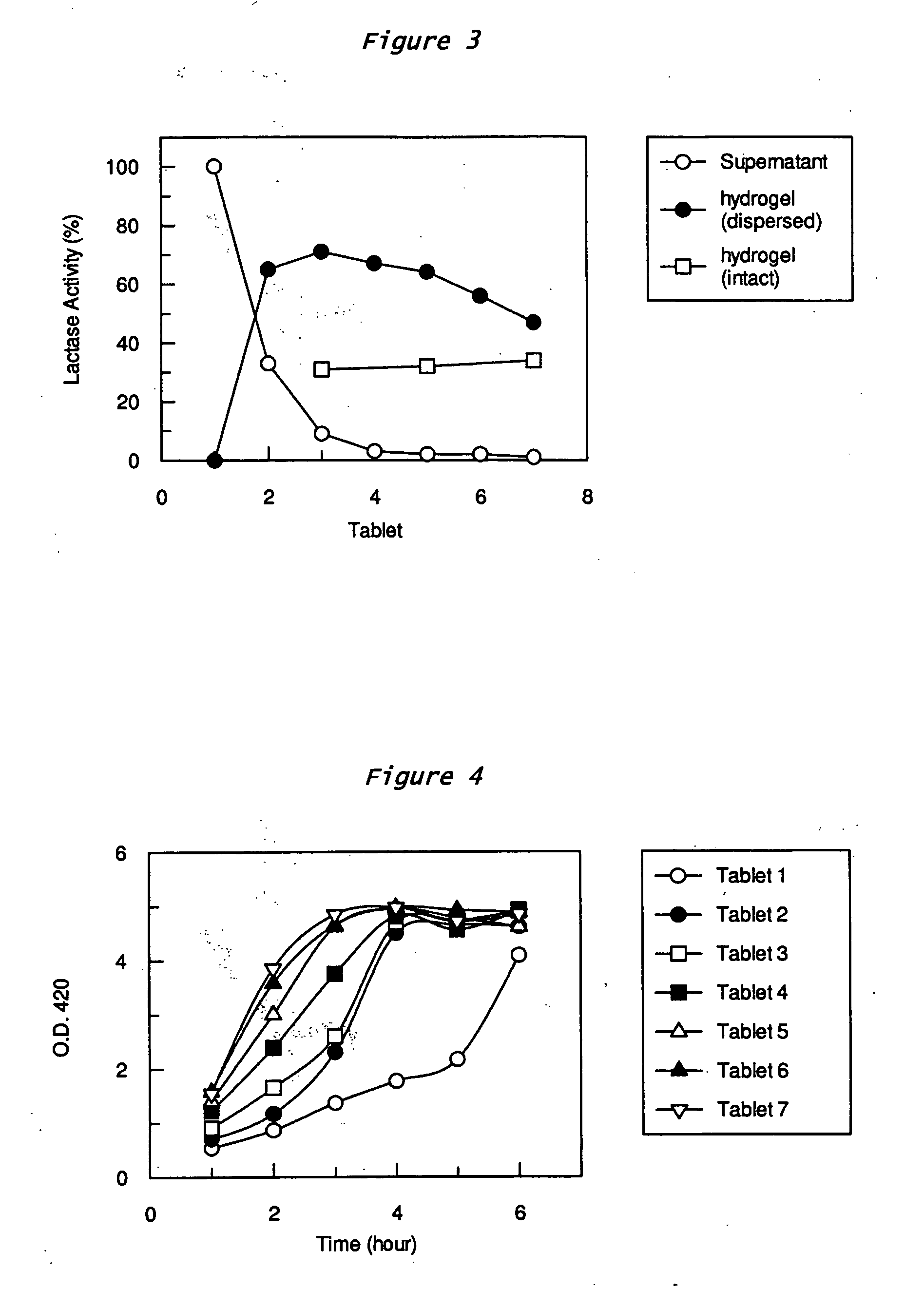
Controlled release formulations of enzymes, microorganisms, and antibodies with mucoadhesive polymers
InactiveUS20080020036A1Easy to exportDisintegrates quicklyPowder deliveryPeptide/protein ingredientsMicroorganismWater soluble
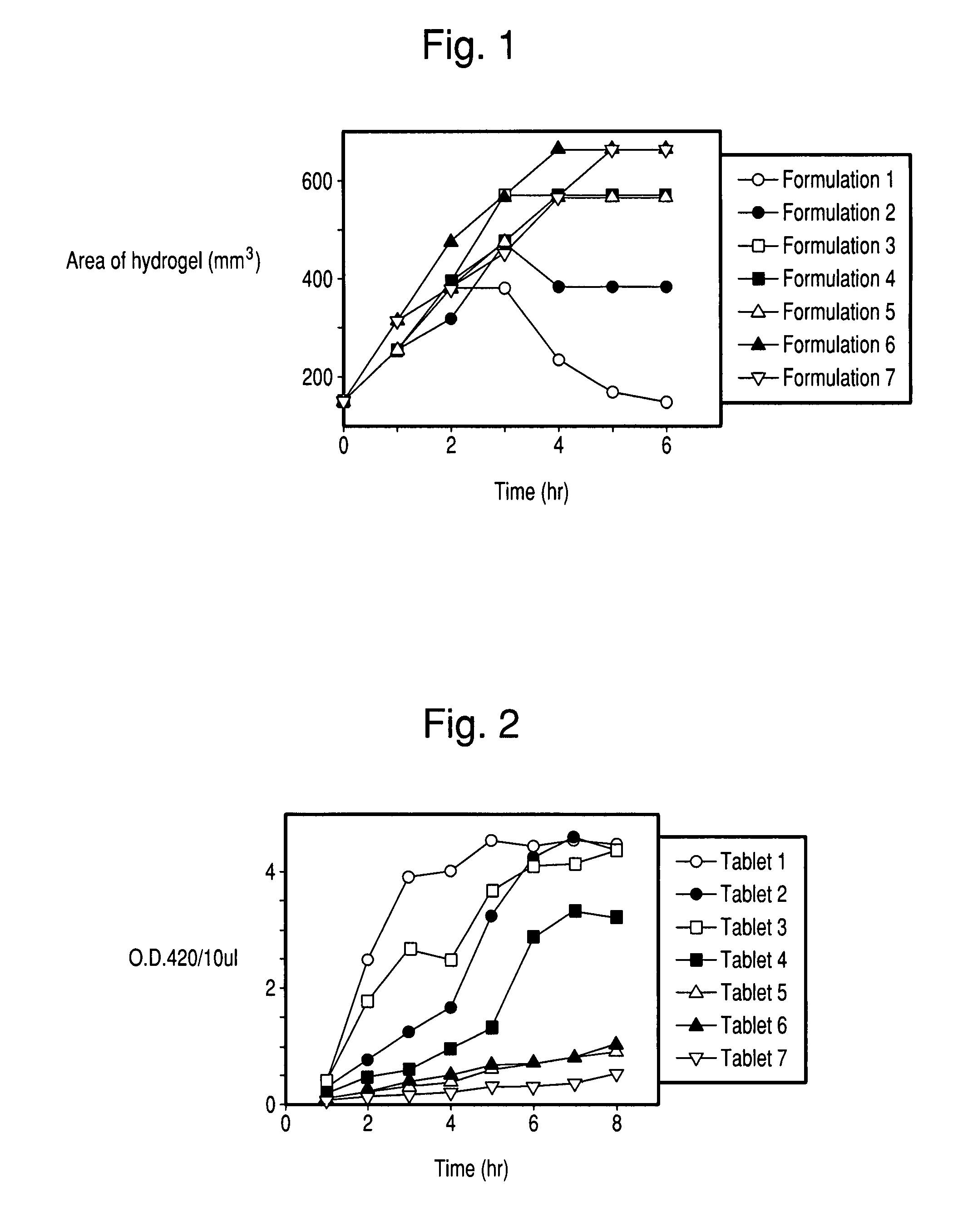
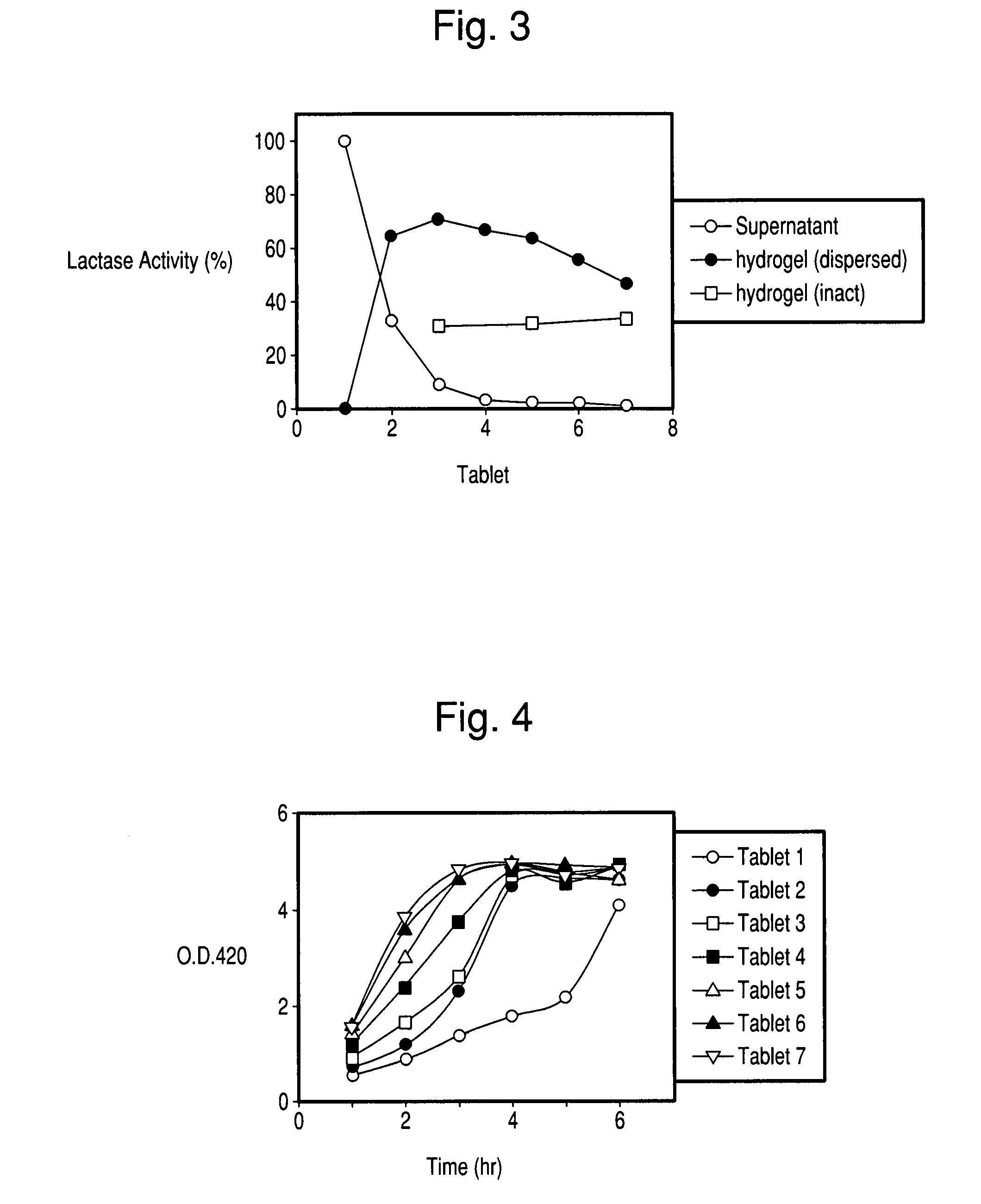
There is provided a composition comprising at least one mucoadhesive polymer that is capable of forming a hydrogel and at one least water soluble polymer, and one or more enzymes, microorganisms, or antibodies. The formulation forms a hydrogel in aqueous solution that has mucoadhesive properties and that is capable of releasing the enzymes, microorganisms, or antibodies over an extended period of time and / or of entrapping enzymes, microorganisms, or antibodies within the hydrogel that is active for an extended time.
Owner:AMANO ENZYME USA CO LTD +1
Therapeutic oral composition
ActiveUS20130078197A1Alleviate hypersensitivityPromote remineralizationAntibacterial agentsCosmetic preparationsArgininePotassium
Disclosed are therapeutic oral compositions useful in the treatment of a variety of oral disorders, in which the composition can provide blockage of dentinal tubes, while at the same time provide antibacterial and anti-caries efficacy. The compositions include arginine in free or salt form, a mucoadhesive polymer, and at least one component selected from pyrophosphates, zinc salts, potassium salts, strontium salts, and mixtures thereof.
Owner:COLGATE PALMOLIVE CO
Mucoadhesive Oral Formulations of High Permeability, High Solubility Drugs
Solid oral dosage formulations, such as tablet, mini-tab, multiparticulates or osmotic delivery systems, are coated with a mucoadhesive polymeric coating or formed of a mucoadhesive polymer to increase oral bioavailability of Biopharmaceutical Classification System (BCS) Class I drugs. Representative BCS I drugs include valacyclovir, gabapentin, furosemide, levodopa, metformin, and ranitidine HCl. The inclusion of mucoadhesives in the solid oral dosage form brings the dosage form into close proximity with the target epithelium and facilitates diffusion of drug into intestinal tissue. The mucoadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. Preferred mucoadhesive polymers include poly(adipic)anhydride “P(AA)” and poly(fumaric-co-sebacic)anhydride “P(FA:SA)”. Other preferred mucoadhesive polymers include non-erodable polymers such as DOPA-maleic anhydride co polymer; isopthalic anhydride polymer; DOPA-methacrylate polymers; and DOPA-cellulosic based polymers.
Owner:JACOB JULES S +4
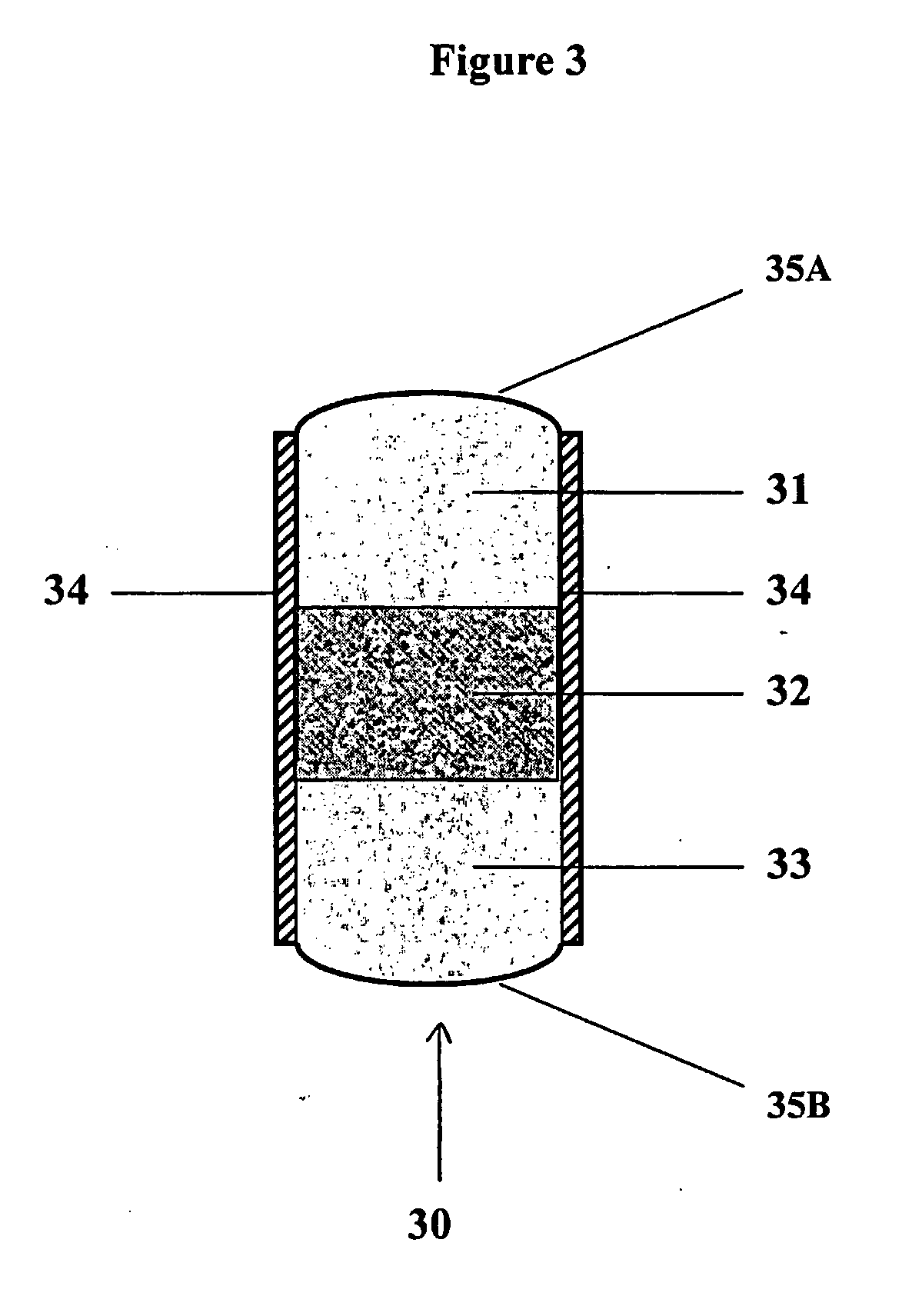
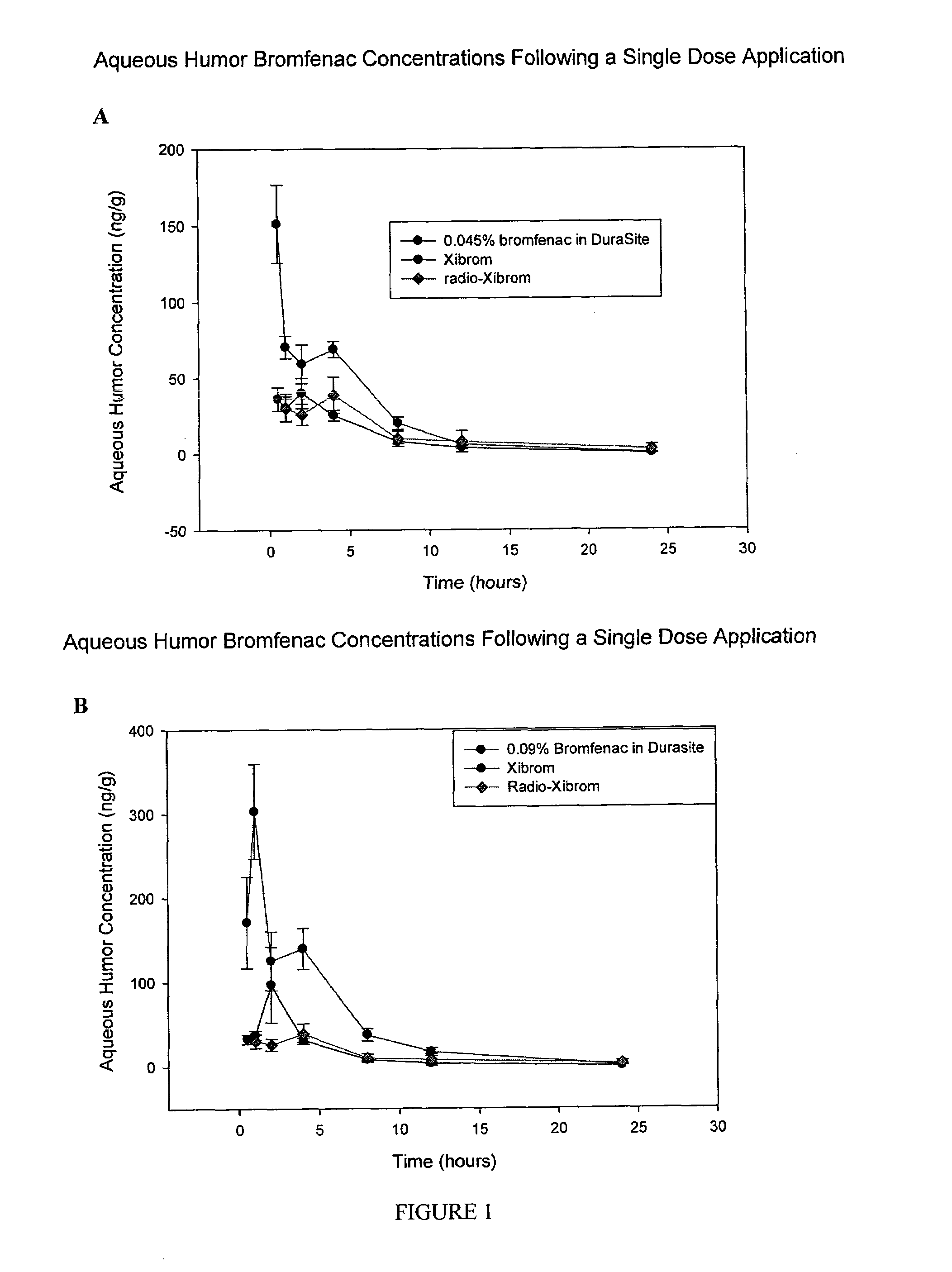
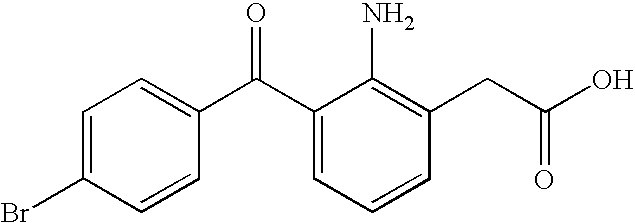
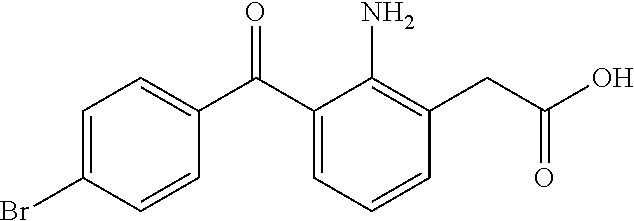
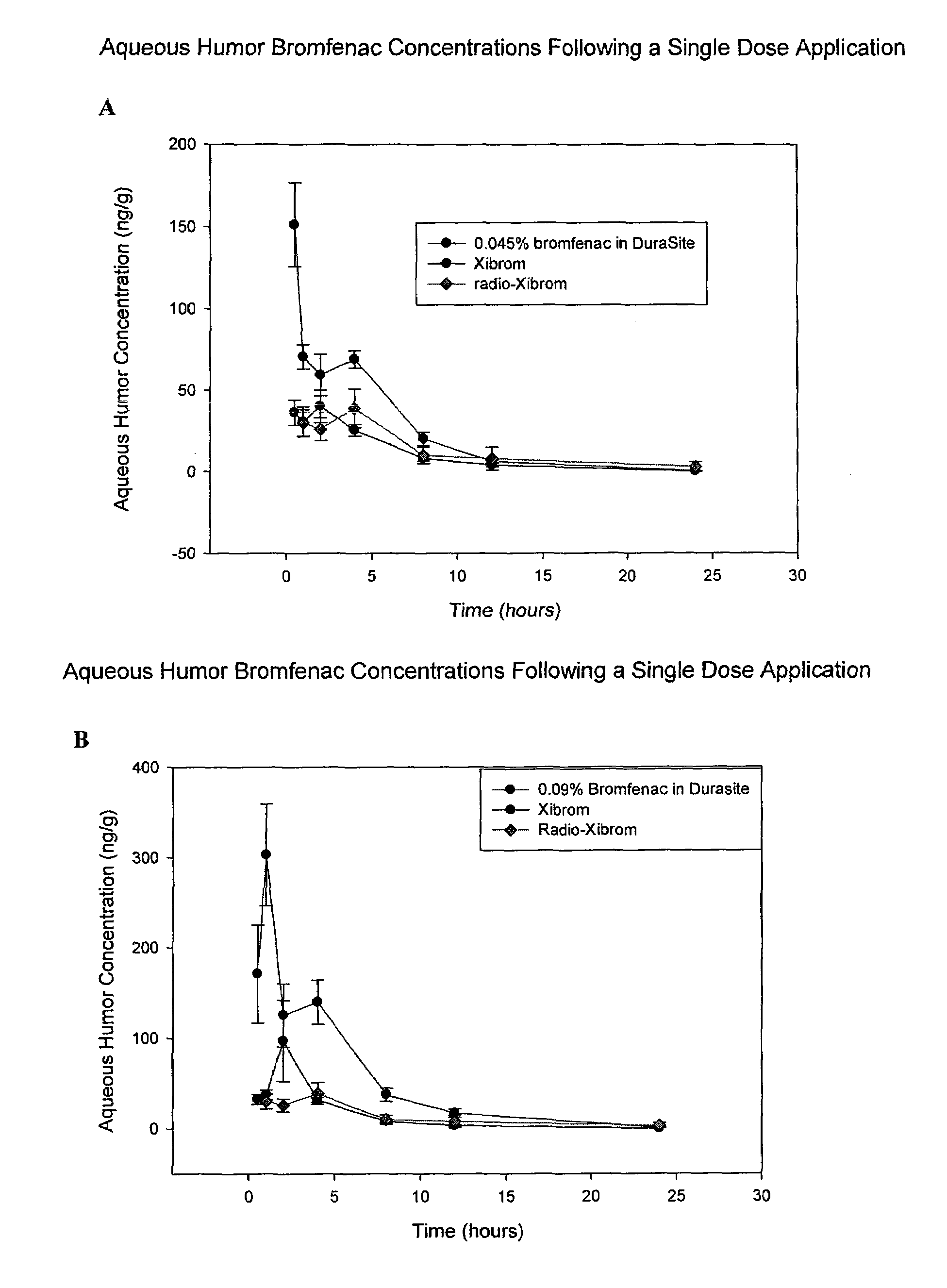
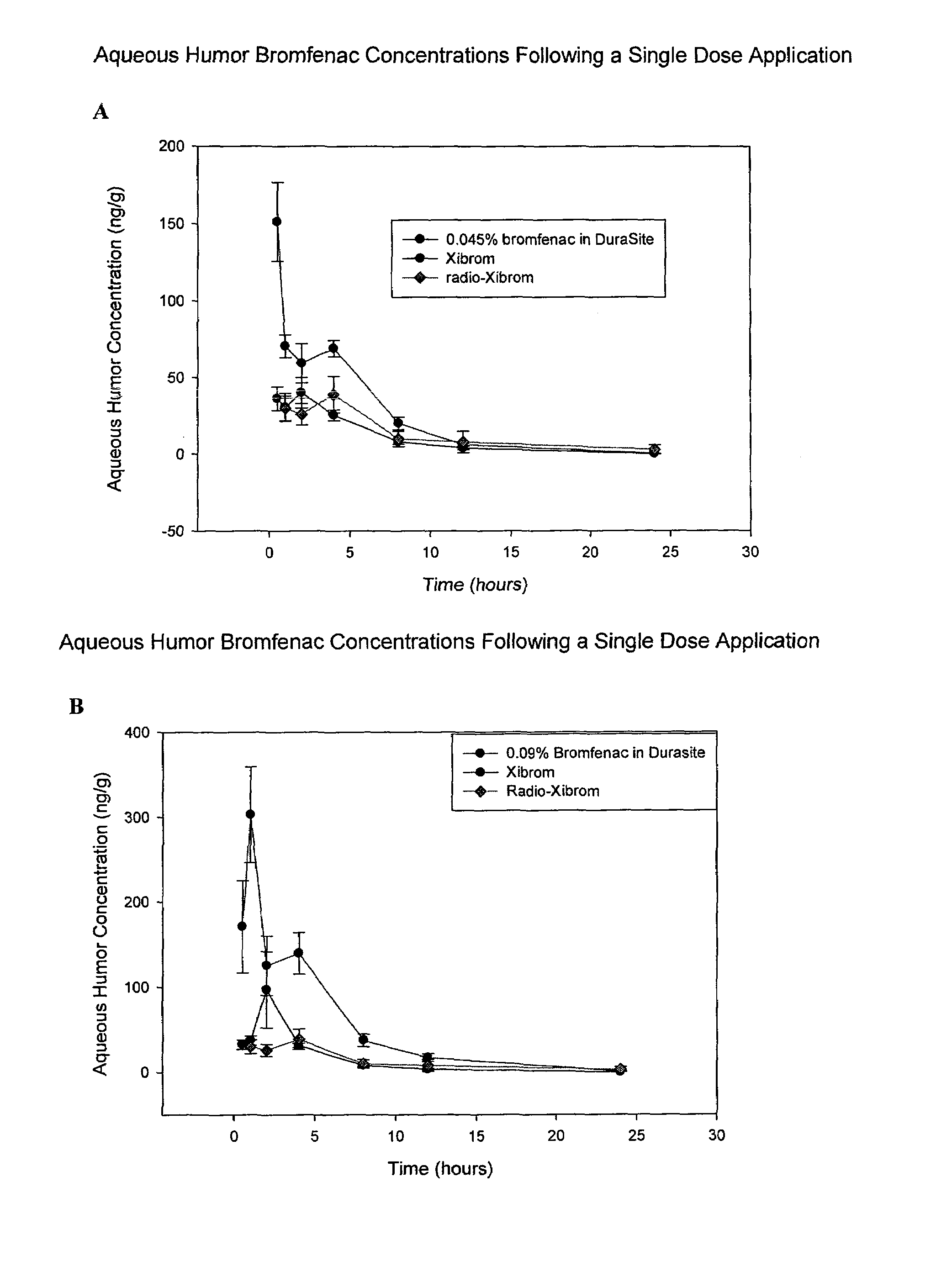
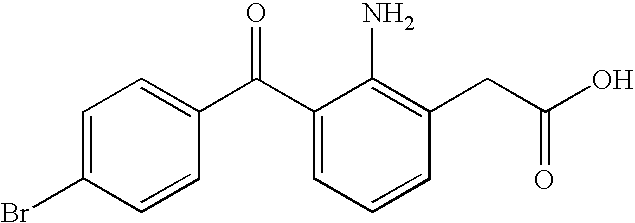
Non-steroidal Anti-inflammatory ophthalmic compositions
The disclosure provides compositions and systems for topical ophthalmic application, which include an aqueous mixture of bromfenac and flowable mucoadhesive polymer, for treating inflammation and inflammatory conditions of the eye.
Owner:SUN PHARMA INDS
Combination Anti-inflammatory ophthalmic compositions
InactiveUS20130165419A1Good anti-inflammatory effectGood slow releaseAntibacterial agentsBiocideOphthalmologyNon steroidal anti inflammatory
Compositions and systems for topical ophthalmic application, which include an aqueous mixture of steroidal and non-steroidal anti-inflammatory agents in a flowable mucoadhesive polymer, for treating inflammation and inflammatory conditions of the eye.
Owner:INSITE VISION
Microparticulated vaccines for the oral or nasal vaccination and boostering of animals including fish
ActiveUS20120040010A1Stabilizes sensitive bioactiveImprove thermal stabilitySsRNA viruses negative-sensePowder deliveryVaccinationAquatic animal
The invention relates to a composition and a method for manufacturing semi-dry or dry particles containing a mucoadhesive polymer and a bioactive agent such as, but not limited to, an Immunogenic Substance (e.g., a vaccine), that allows the oral or nasal administration and delivery of the bioactive agent essentially unaltered to mucosal surfaces in the animal, including an aquatic animal.
Owner:INTERVET INC
Ph-responsive mucoadhesive polymeric encapsulated microorganisms
ActiveUS20170165201A1Improve survivabilityHigh retention rateBacterial antigen ingredientsViral antigen ingredientsAntigenMicroorganism
Methods of encapsulating microorganisms, or components thereof, for targeted enteric delivery to an animal host have been developed. In particular, the encapsulation provides a prolonged survival, extended retention and a pH-sensitive release of the encapsulated microorganisms or antigenic components thereof at targeted sites within the gastrointestinal tract. The formulations are useful for diagnostic, therapeutic and prophylactic purposes and can alter a host's microbial composition associated with a condition or a disease state.
Owner:MASSACHUSETTS INST OF TECH
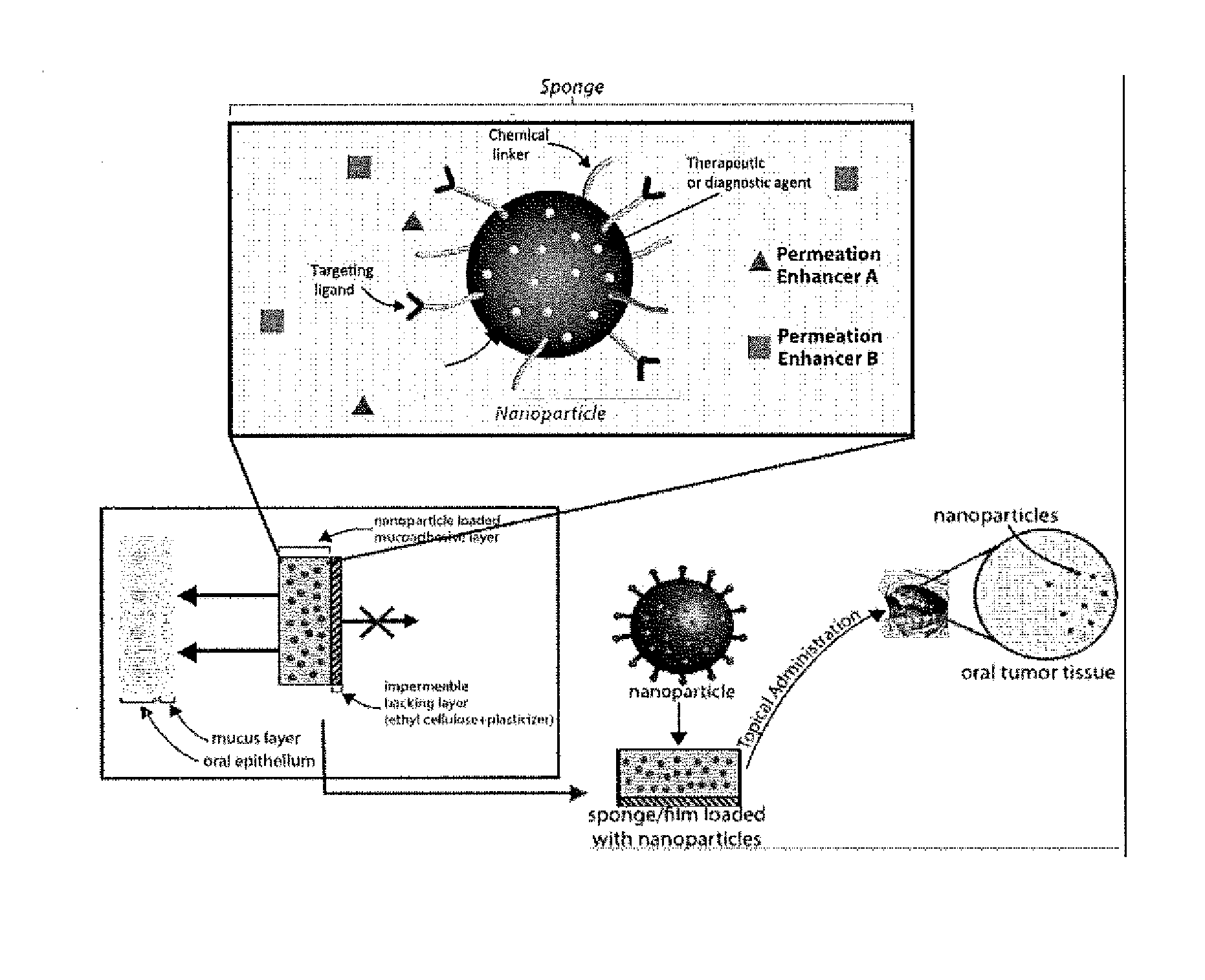
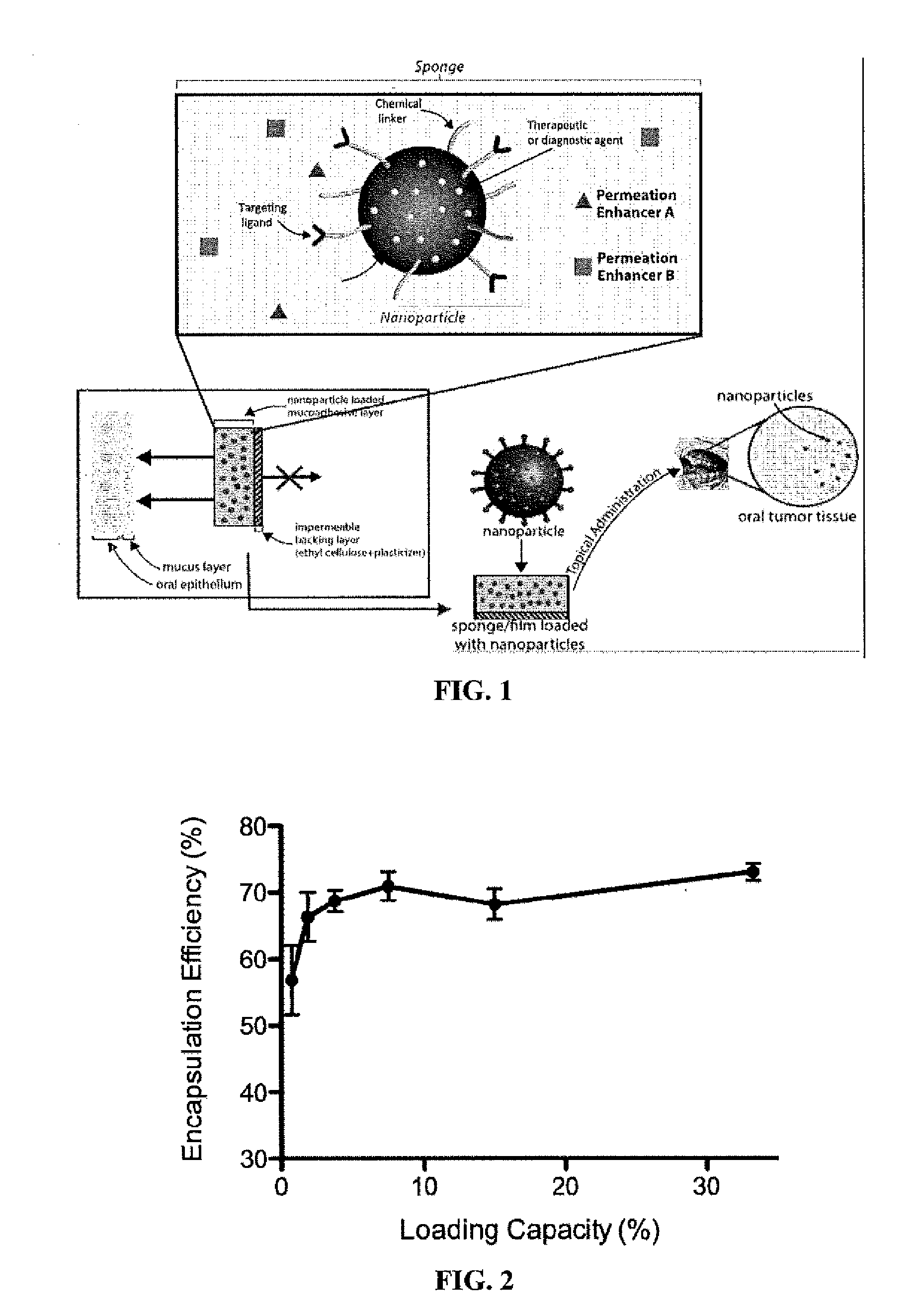
Targeted Buccal Delivery of Agents
InactiveUS20140234212A1Avoid bitternessPrevent unpleasant tasteAntibacterial agentsHeavy metal active ingredientsCancer cellWhole body
A delivery device for topical and systemic delivery of agents to targeted oral locations, such as mouth cancer cells, has been developed. The formulation includes a mucoadhesive polymeric matrix such as chitosan, which contains one or more therapeutic and / or diagnostic agents, taste masking agents, permeation enhancers, the therapeutic or diagnostic agent to be delivered, and a hydrophilic polymeric coating such as polyethyleneglycol (“PEG”). In the preferred embodiment, the matrix is formulated with one side having the PEG-mucoadhesive polymer exposed for topical placement onto epithelial or cancer cells in the mouth or other mucosal area and the side(s) facing the inside of the oral cavity being covered with a biocompatible, inert membrane that is impermeable to the therapeutic and / or diagnostic agent(s) to be delivered.
Owner:MASSACHUSETTS INST OF TECH
Oral mucoadhesive dosage form
ActiveUS8735374B2Improve stabilityPromote absorptionOrganic active ingredientsBiocideSolubilityLiquid medium
A direct compression formulation suitable for preparing buccal and / or sublingual and dosage forms incorporates a combination of a non-ionic polymeric solubility enhancer, a mucoadhesive polymer, a filler, a disintegrant, and a pharmaceutically active agent. Cannabinoid-cyclodextrin complexes exhibiting an improved property selected from improved stability, higher product yield and improved product uniformity may be obtained by complexing the cannabinoid with the cyclodextrin in a liquid medium containing an antioxidant. To enhance stability, product yield and / or product uniformity, complexing may be done while the liquid medium is in contact with an atmosphere having a very low oxygen content. The resulting complexes may be combined with decomplexing agents and / or dispersed in a matrix material comprised of a hydrogel-forming polymer to provide enhanced absorption of the cannabinoid through oral mucosa and reduced ingestion of the cannabinoid as compared with known commercially available cannabinoid-containing oral dosage forms.
Owner:INTELGENX CORP
Therapeutic oral composition
ActiveUS9579269B2Ameliorate dry mouthTreat erosion and gingivitisAntibacterial agentsCosmetic preparationsArginineStrontium
Disclosed are therapeutic oral compositions useful in the treatment of a variety of oral disorders, in which the composition can provide blockage of dentinal tubes, while at the same time provide antibacterial and anti-caries efficacy. The compositions include arginine in free or salt form, a mucoadhesive polymer, and at least one component selected from pyrophosphates, zinc salts, potassium salts, strontium salts, and mixtures thereof.
Owner:COLGATE PALMOLIVE CO
Sublingual apomorphine
ActiveUS9044475B2Alleviating dyskinesiaEffectively alleviatedBiocidePowder deliveryMedicineSexual dysfunction
The invention features sublingual formulations of apomorphine that is a mucoadhesive polymer film or a strip having a first portion including an acid addition salt of apomorphine and a second portion including a pH neutralizing agent, and methods of treating Parkinson's disease, sexual dysfunction, and depressive disorders by administering sublingually the film or strip.
Owner:SUNOVION PHARMA INC
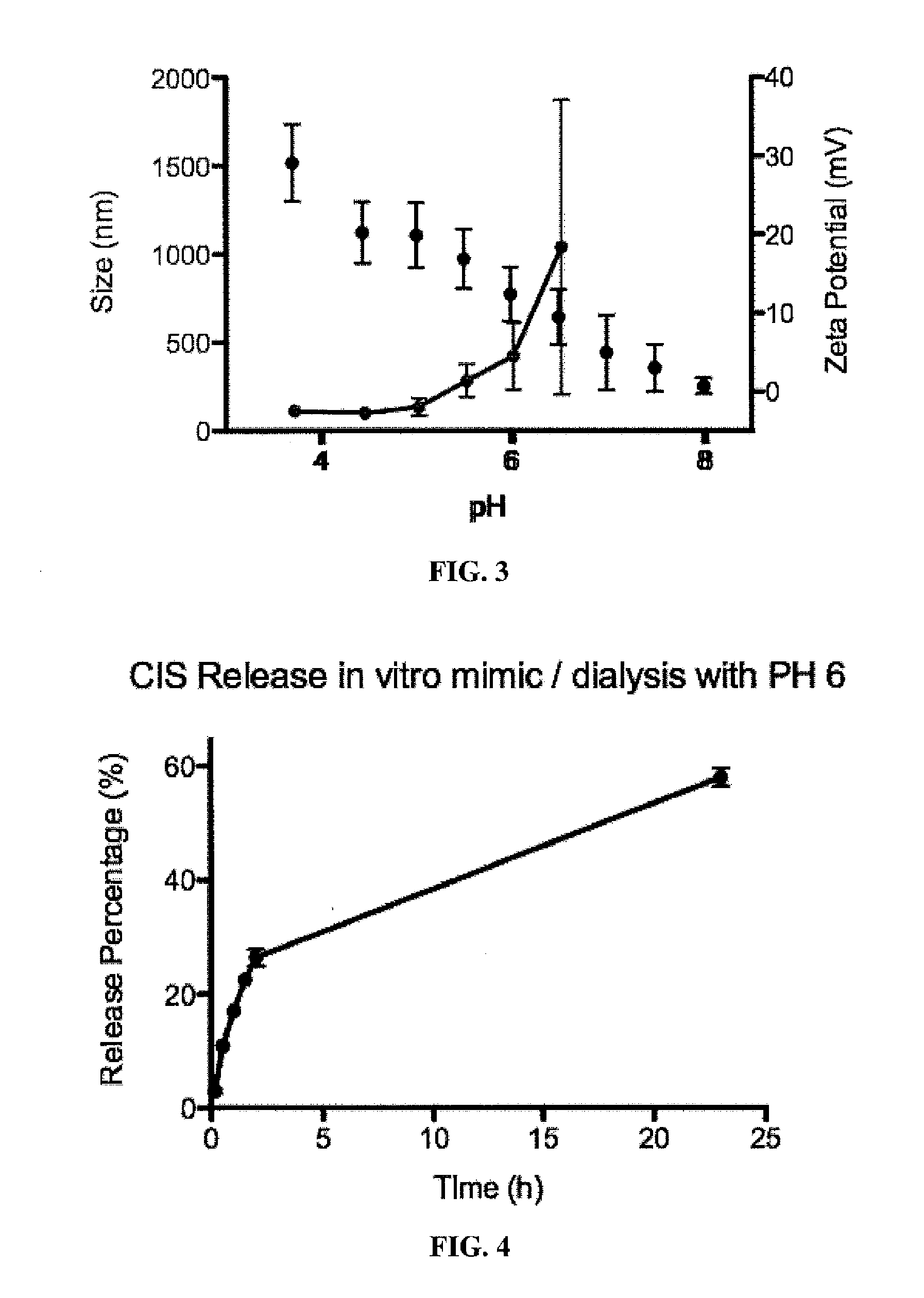
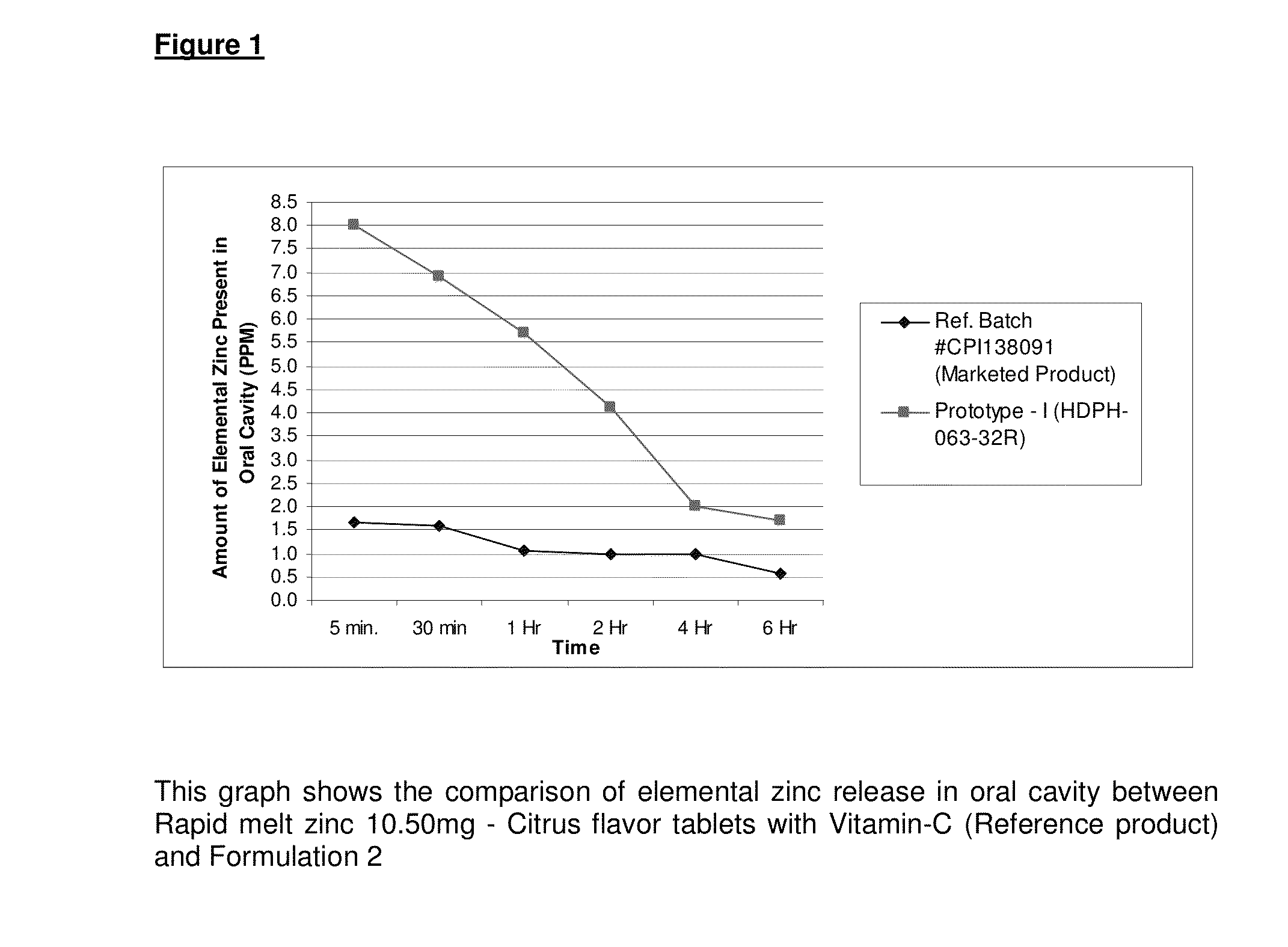
Quick Dissolving, Long Acting Zinc Therapeutic Formulations
InactiveUS20130039981A1Reduce in quantityGood curative effectBiocideNervous disorderControl releaseBioadhesive
The present invention comprises a quick dissolving, long acting zinc therapeutic cold formulation containing high levels of an active compound encapsulated within bioadhesive / muco-adhesive polymers as a controlled release oral drug delivery system. The composition allows for increased residence time for enhanced prophylactic and therapeutic efficacy within the mouth and oral cavity. This allows for a reduction in the number of doses necessary to achieve therapeutic relief which will result in increased patience compliance.
Owner:CHERURKURI SUBRAMAN RAO
Controlled Surface Gelling of Mucoadhesive Polymers on Oral Mucosa
The present invention relates to an oral composition and method for alleviating the symptoms associated with xerostomia using encapsulated cation-releasing compounds formulated either intimately together or in separate compartments in a composition containing cation-sensitive mucoadhesive polymers.
Owner:COLGATE PALMOLIVE CO
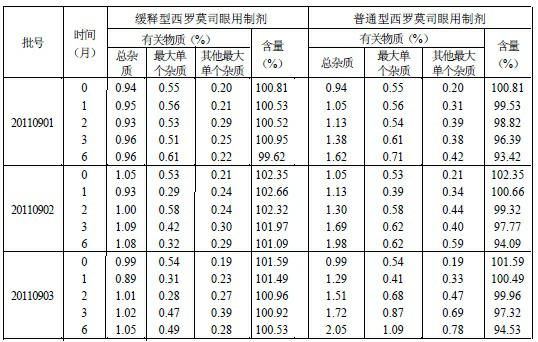
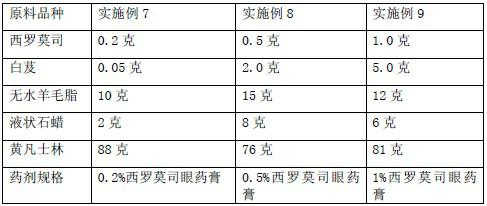
Slow-release sirolimus ophthalmic preparation
ActiveCN102670499ASolve solubilityFix stability issuesOrganic active ingredientsSenses disorderOphthalmologyMucoadhesive polymers
The invention relates to an external slow-release sirolimus ophthalmic preparation for ophthalmology, which is prepared by sirolimus as a pharmacodynamic raw material, bletilla striata as a slow release formulation, and appropriate excipients according to a ratio of sirolimus to bletilla striata of 1.0: (0.1 to 10.0). According to the external slow-release sirolimus ophthalmic preparation for the ophthalmology, the bletilla striata is used as a mucoadhesive polymer drug release system, so that the problems that solubility and stability of the sirolimus in a pure aqueous solution are poor are solved; and the convergence, hemostasis, heat clearing and diuresis promoting, detumescence and granulation promoting effects of the bletilla striata are utilized to enhance the immunosuppressive effect and the eye tissue penetration of the sirolimus.
Owner:GUANGDONG WHOLEWIN TECH
Enteric-coated capsule containing cationic nanoparticles for oral insulin delivery
ActiveCN102908332APromote absorptionPowder deliveryPeptide/protein ingredientsNanoparticleSurface charges
The invention relates to an enteric-coated capsule containing cationic nanoparticles for oral insulin delivery, in particular to a type of cationic nanoparticle including a polycationic and mucoadhesive polymer and a biodegradable polymer, wherein each of the nanoparticles has positive surface charge and enhanced permeability for paracellular insulin delivery; the enteric-coated capsule further includes a pH-sensitive polymer as the coating. The enteric-coated capsule containing cationic nanoparticles, when being orally administered to a subject, are configured to prevent the acidic degradation of the active substance such as insulin before being released from said cationic nanoparticles to a specific absorption site along the gastrointestinal tract.
Owner:NANO & ADVANCED MATERIALS INST
Conjugate comprising pharmaceutical active compound covalently bound to mucoadhesive polymer and transmucosal delivery method of pharmaceutical active compound using the same
InactiveCN101405301AGood yieldGood biocompatibilityPeptide/protein ingredientsPharmaceutical non-active ingredientsDiseaseBiocompatibility Testing
Owner:GWANGJU INST OF SCI & TECH
Controlled release formulations of enzymes, microorganisms, and antibodies with mucoadhesive polymers
InactiveUS20050281795A1Satisfies needDigestionPeptide/protein ingredientsBacteria material medical ingredientsMicroorganismWater soluble
There is provided a composition comprising at least one mucoadhesive polymer that is capable of forming a hydrogel and at one least water soluble polymer, and one or more enzymes, microorganisms, or antibodies. The formulation forms a hydrogel in aqueous solution that has mucoadhesive properties and that is capable of releasing the enzymes, microorganisms, or antibodies over an extended period of time and / or of entrapping enzymes, microorganisms, or antibodies within the hydrogel that is active for an extended time.
Owner:AMANO ENZYME USA CO LTD +1
Oral care product for sensitive enamel care
InactiveUS20120308488A1Avoid erosionReduce bacterial adhesionCosmetic preparationsGum massageMouth careTooth enamel
Owner:COLGATE PALMOLIVE CO
Non-steroidal anti-inflammatory ophthalmic compositions
ActiveUS8778999B2Promote degradationOptimal in flowabilityBiocideSenses disorderOphthalmologyBromfenac
The disclosure provides compositions and systems for topical ophthalmic application, which include an aqueous mixture of bromfenac and flowable mucoadhesive polymer, for treating inflammation and inflammatory conditions of the eye.
Owner:SUN PHARMA INDS
Mucoadhesive thermoresponsive medicament-carrier composition
InactiveUS20070231352A1The drug effect is goodLittle side effectsPeptide/protein ingredientsEnergy modified materialsAmino-Levulinic AcidMedicine
This invention relates to a medicament-carrier composition for use in delivering medicaments or fixing the action site of biological active compounds, which mainly comprises a mucoadhesive polymer and a thermoresponsive polymer. The medicament-carrier composition according to this invention is quite suitable for use in topically delivering biological active compounds, especially those useful in photodynamic diagnosis or therapy, e.g. 5-aminolevulinic acid (called as “ALA” for short).
Owner:PHARMA POWER BIOTEC
Methods and biocompatible compositions to achieve sustained drug release in the eye
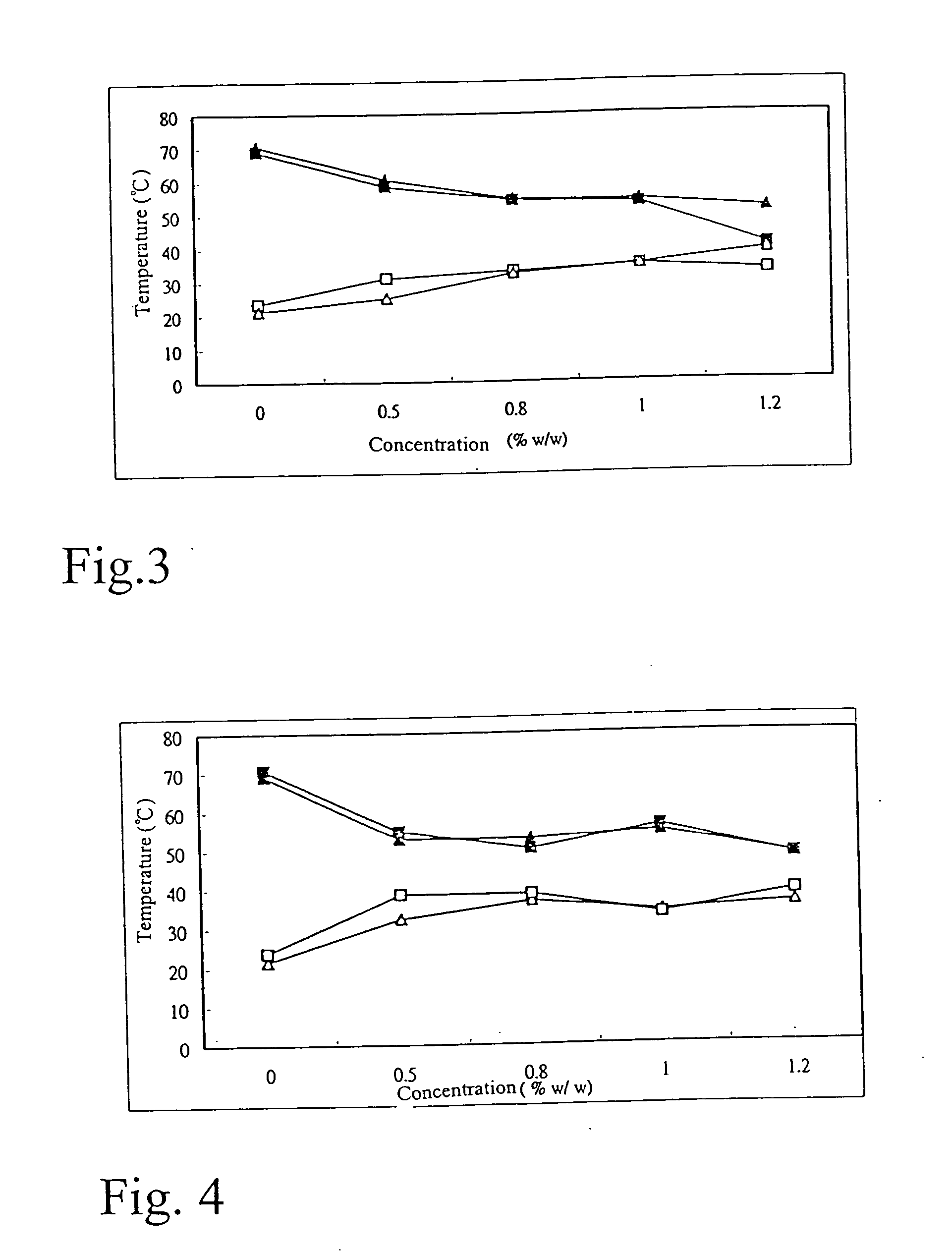
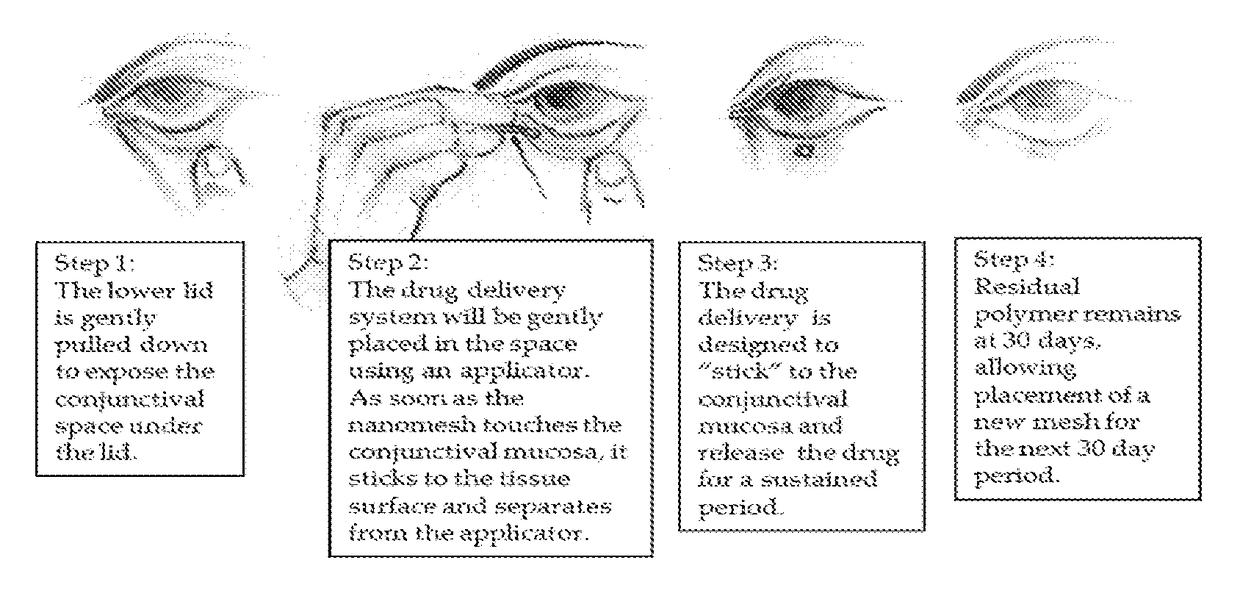
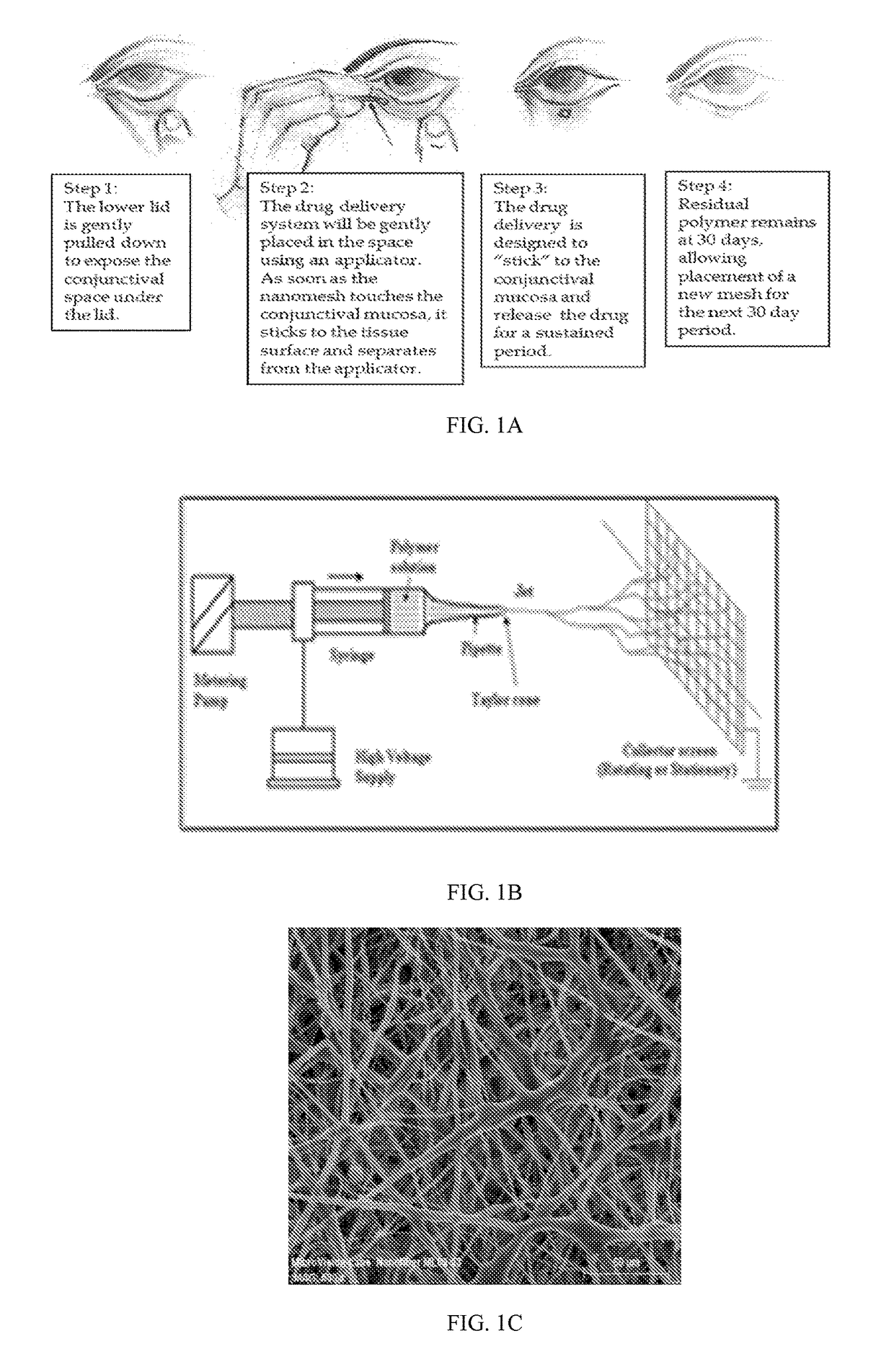
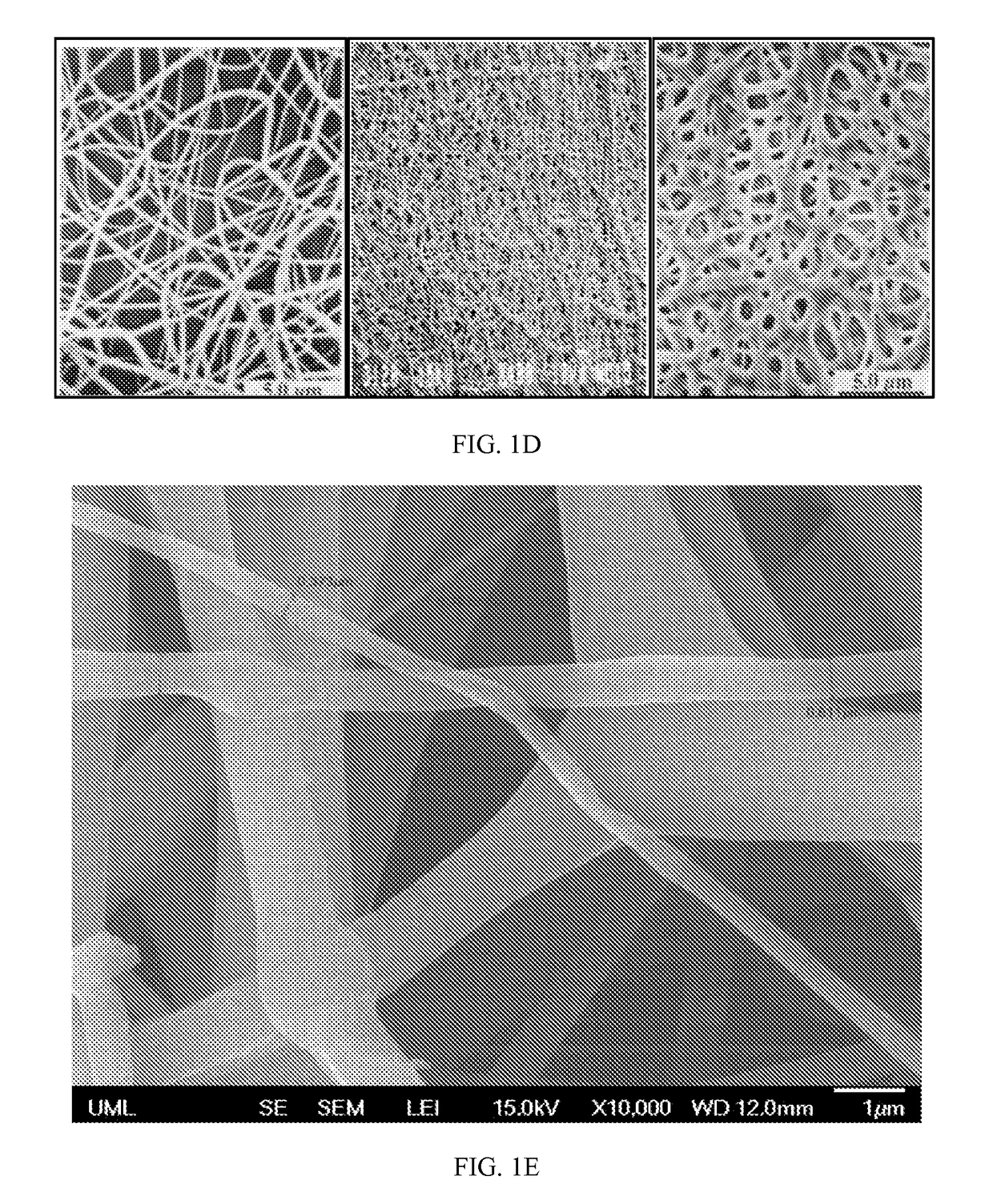
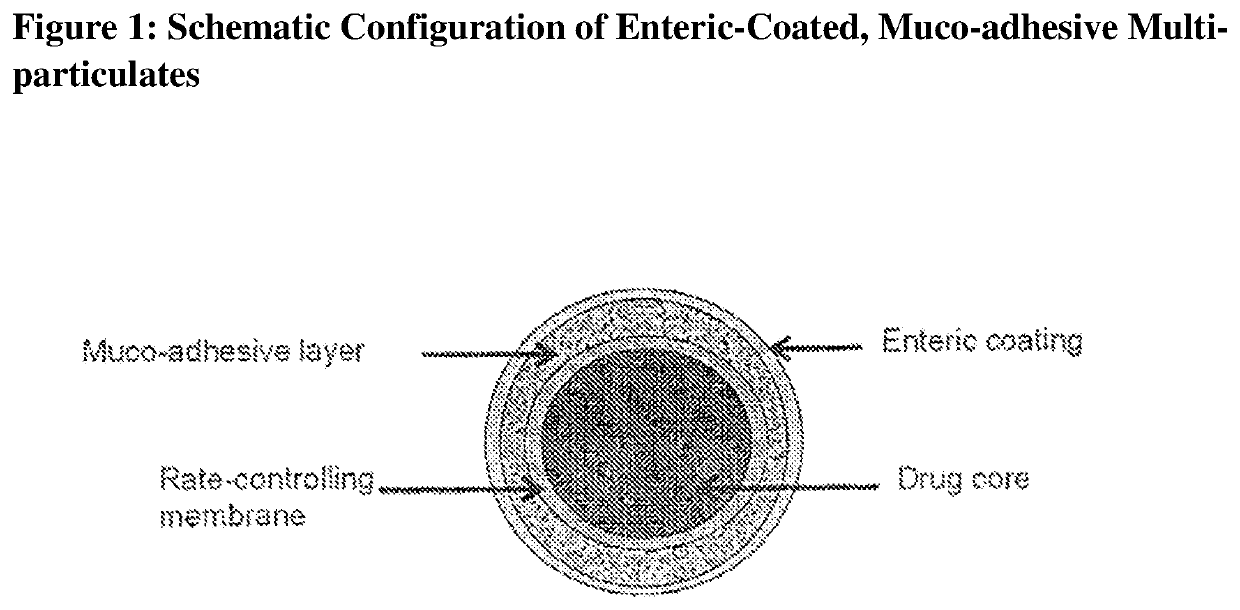
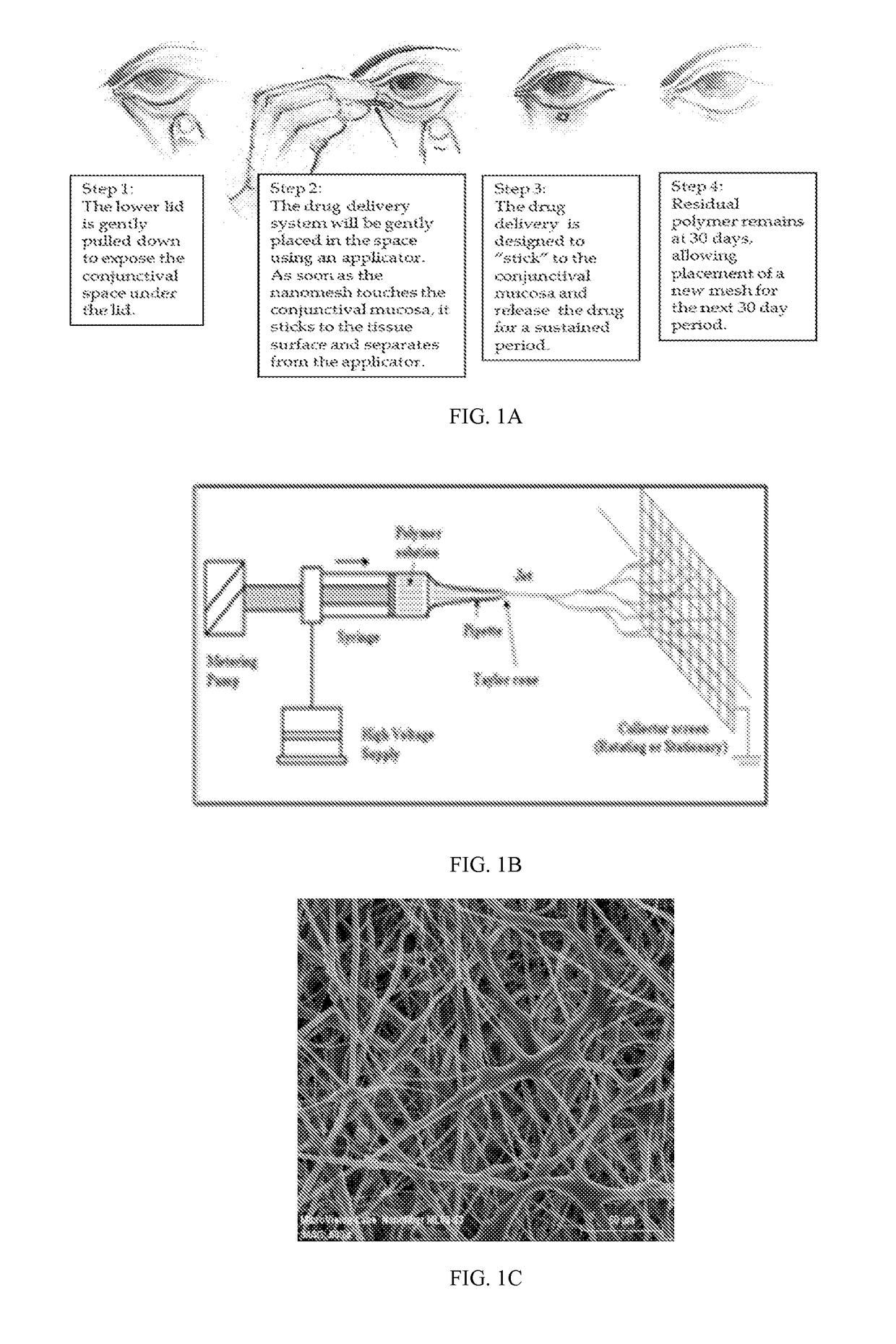
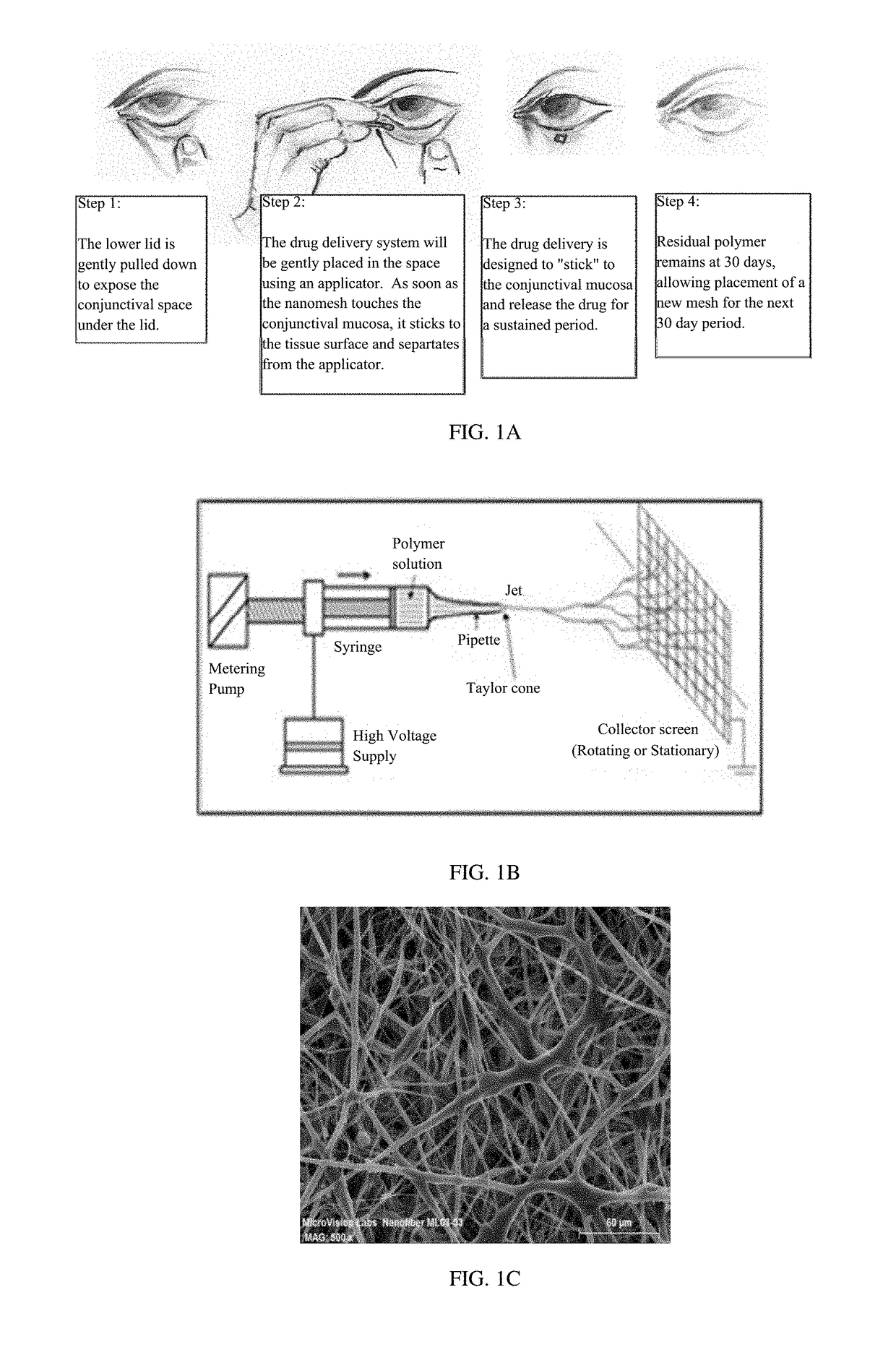
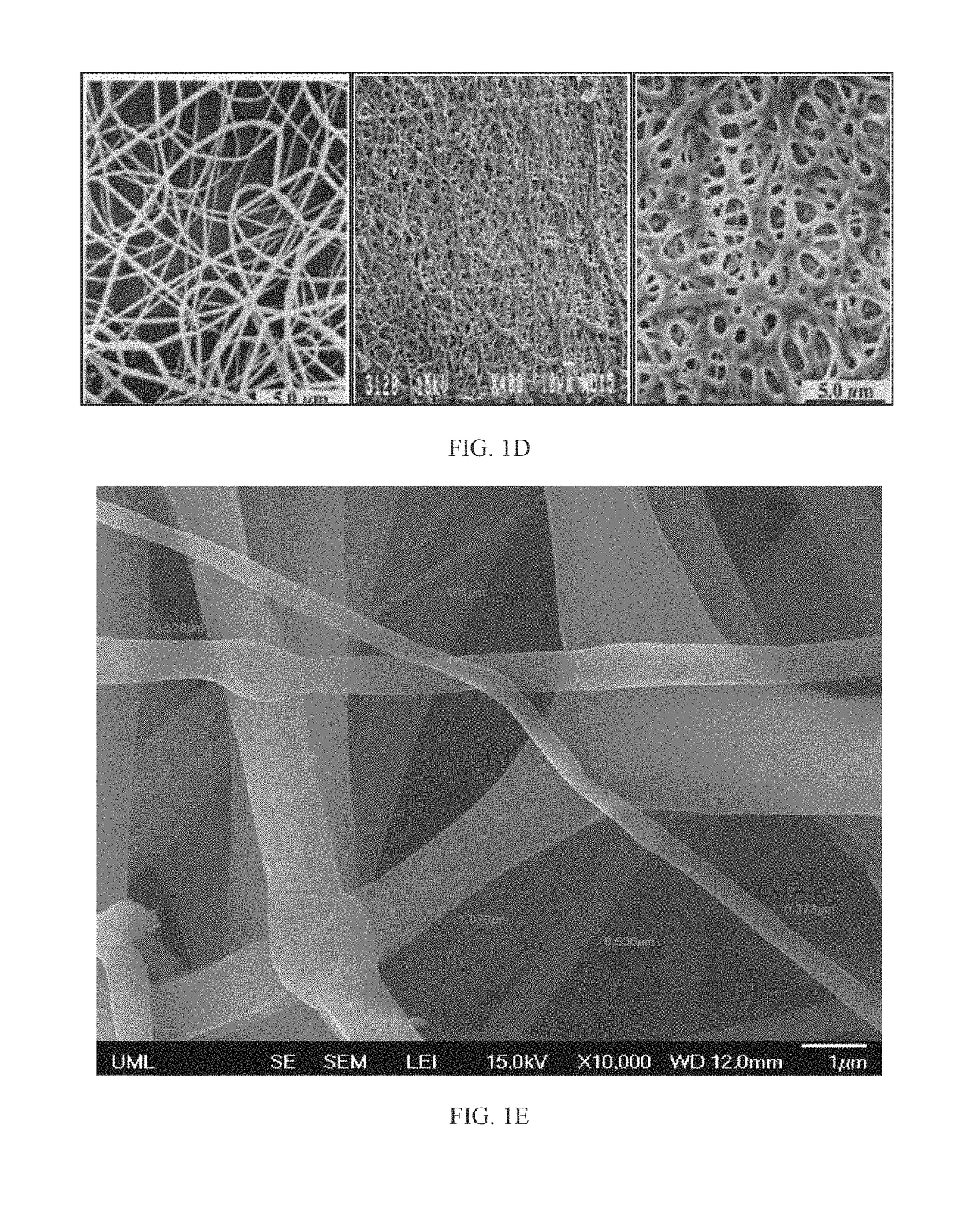
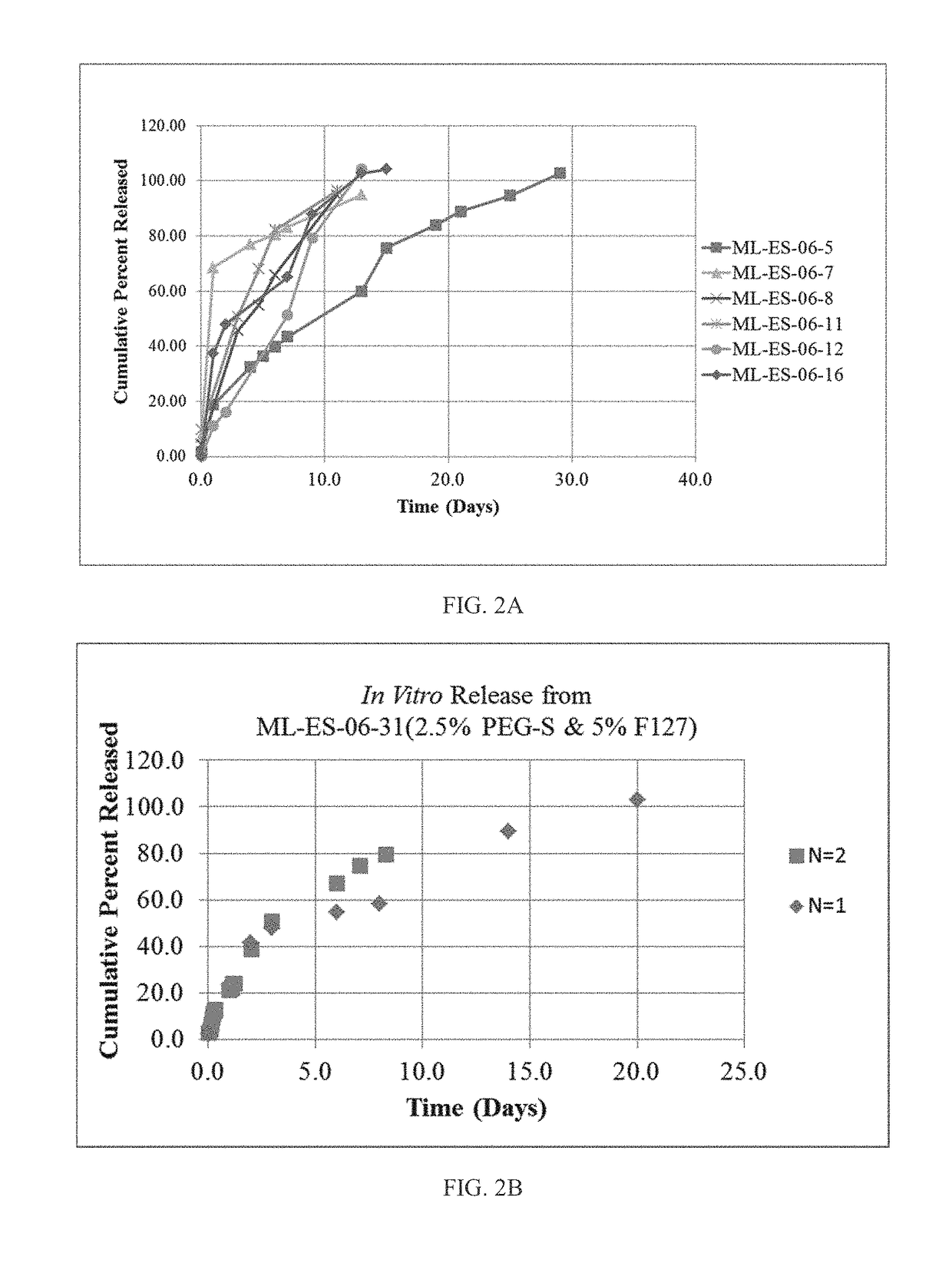
A nanostructured biocompatible wafer for placement in the conjunctival cul-de-sac. The wafer contains a tissue-reactive mucoadhesive polymer and a mesh formed of a plurality of hydrophobic polymer fibers. Also provided is a method for treating glaucoma, an ocular surface disorder, or an ocular surface infection using the nanostructured biocompatible wafer. Additionally, an injectable sustained-release formulation for treating an ocular disorder is disclosed. The formulation includes a drug contained within a plurality of microparticles formed of a biodegradable polymer and are coated with a tissue-reactive compound. Further provided is a method for treating an ocular disorder by injecting the microparticulate sustained release formulation.
Owner:INTEGRAL BIOSYST
Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof
ActiveUS10987313B2Effective amountOrganic active ingredientsGranular deliveryMedicinal chemistryEnzyme inhibitor
The invention provides a controlled release oral solid formulation comprising (a) a controlled release component comprising core comprising levodopa and / or an ester of levodopa or salts thereof, wherein the core is coated with a layer of a muco-adhesive polymer and externally coated with a layer of an enteric coated polymer; and (b) a decarboxylase inhibitor component.
Owner:IMPAX LAB LLC
Ophthalmic emulsion
ActiveUS20160338952A1Small mean dropletEmulsion stabilizationOrganic active ingredientsSenses disorderOcular surfacePolymer
The present invention is directed to an ophthalmic emulsion. The emulsion has a unique combination of ingredients that promotes the stability of small oil droplets within the emulsion. The emulsion also includes a mucoadhesive polymer that aid in delivering a lipid to the ocular surface.
Owner:ALCON INC
Methods and biocompatible compositions to achieve sustained drug release in the eye
A nanostructured biocompatible wafer for placement in the conjunctival cul-de-sac. The wafer contains a tissue-reactive mucoadhesive polymer and a mesh formed of a plurality of hydrophobic polymer fibers. Also provided is a method for treating glaucoma, an ocular surface disorder, or an ocular surface infection using the nanostructured biocompatible wafer. Additionally, an injectable sustained-release formulation for treating an ocular disorder is disclosed. The formulation includes a drug contained within a plurality of microparticles formed of a biodegradable polymer and are coated with a tissue-reactive compound. Further provided is a method for treating an ocular disorder by injecting the microparticulate sustained release formulation.
Owner:INTEGRAL BIOSYST
Methods and biocompatible compositions to achieve sustained drug release in the eye
A nanostructured biocompatible wafer for drug delivery to a tissue. The wafer contains a tissue-reactive mucoadhesive polymer and a mesh formed of a plurality of polymer fibers. Also provided is a method for treating an ocular surface disease, disorder, or infection using the nanostructured biocompatible wafer. Additionally, an injectable sustained-release formulation for treating an ocular disorder is disclosed. The formulation includes a drug contained within a plurality of microparticles formed of a biodegradable polymer and are coated with a tissue-reactive compound. Further provided is a method for treating an ocular disorder by injecting the microparticulate sustained release formulation.
Owner:INTEGRAL BIOSYST
Micelles for Mucoadhesive Drug Delivery
Biocompatible block copolymer micelles for use in mucoadhesive drug delivery are provided. The micelles comprise a degradable hydrophobic polymer, a degradable synthetic hydrophilic polymer and a mucoadhesive polymer. The micelles are particularly useful for ophthalmic uses.
Owner:MCMASTER UNIV
Composition for enhancing absorption of a drug and method
A composition for enhancing absorption of a pharmaceutical which may have poor oral bioavailability, which composition has surprisingly little cytotoxicity, is provided which is in the form of a liquid or semi-solid or solid containing an admixture (1) a mucoadhesive polymer which is a polyacrylic acid polymer, preferably Carbopol 971P, and (2) an absorption or permeation enhancer which preferably is L-α-lyso-phosphatidylcholine (LPC), and which composition is free of polysaccharides. A method for improving bioavailability of a drug which has poor absorption properties is also provided wherein the above bioadhesive composition is administered with said pharmaceutical to the mucosal membrane of the GI tract, nose, oral cavity, sublingual, buccal, and vaginal mucosa.A method for reducing the cytotoxic effect of an absorption enhancer such as LPC is also provided wherein a mucoadhesive polymer as described above is administered with the LPC to a patient in need of treatment.
Owner:BRISTOL MYERS SQUIBB CO
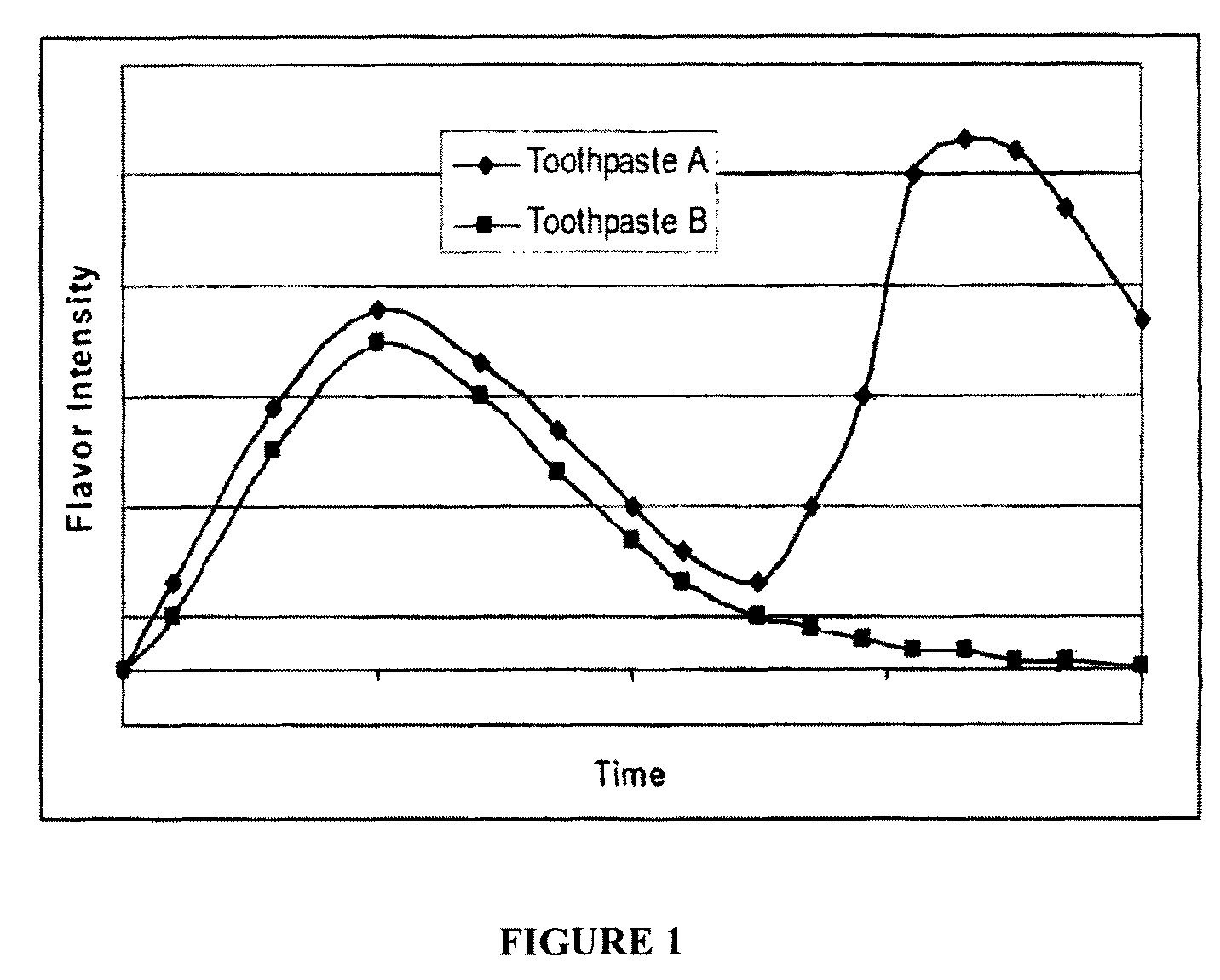
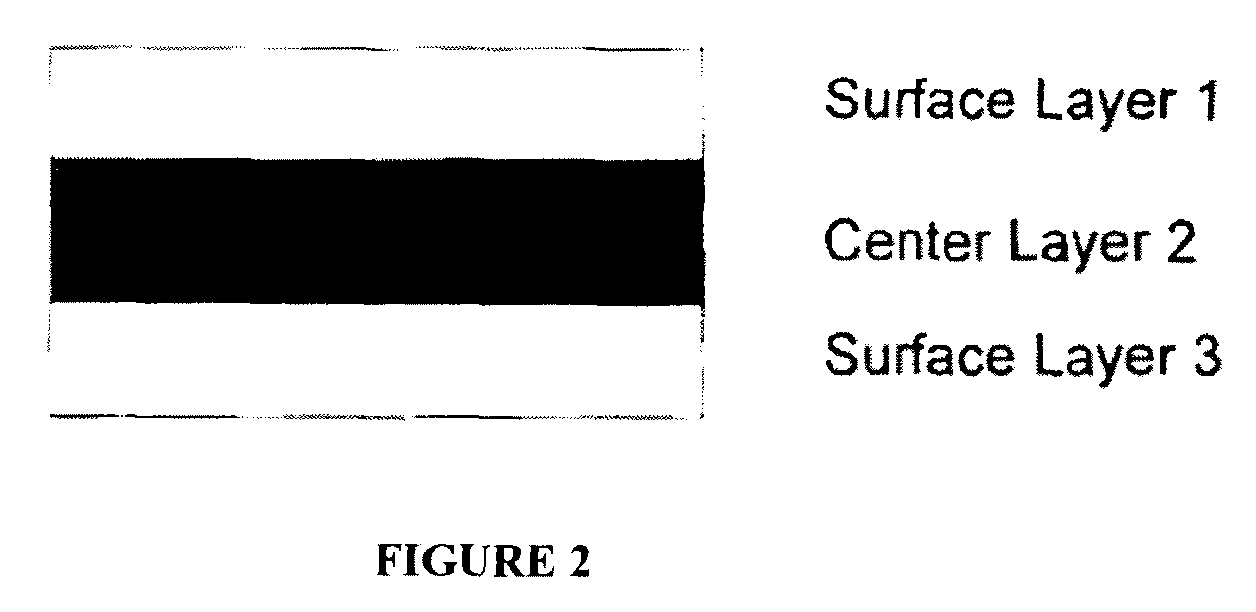
Multilayer films for delivery of flavor
ActiveUS9161890B2Enhance and prolong and delay flavor deliveryEnhanced and prolonged flavor deliveryCosmetic preparationsToilet preparationsSurface layerPolyvinyl acetate
An oral care composition having enhanced flavor release comprising an orally acceptable carrier containing a first flavor and a multilayer film for extended or delayed flavor release, the multilayer film including at least a center layer containing a second flavor, the center layer positioned between two outer surface layers, each surface layer including a release modulating agent, the first and second flavor being the same or different. The film is adapted to adhere to an oral cavity surface and the outer surface layers may comprise a mucoadhesive polymer, e.g. carboxy polymethylene, polycarbophil or polyvinyl pyrrolidone, and a film forming polymer, eg. hydroxypropylmethyl cellulose (HPMC). Suitable release modulating agents include HPMC and polyvinyl acetate. The carrier may be a toothpaste or mouthwash.
Owner:COLGATE PALMOLIVE CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com