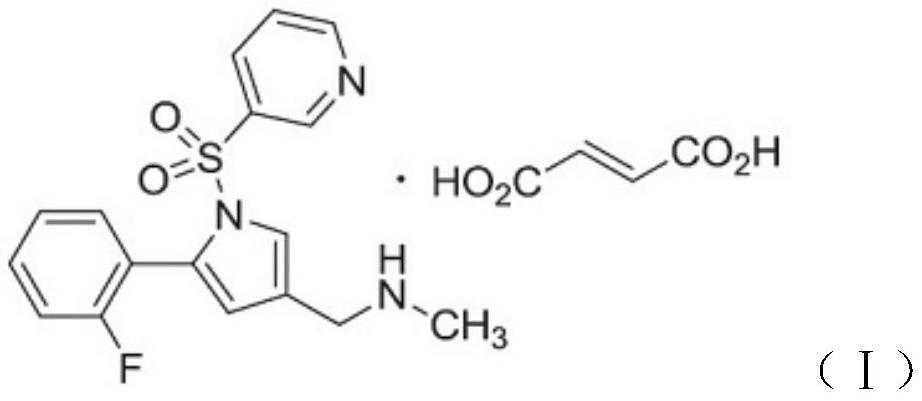
Vonoprazan fumarate-containing tablet and determination method for dissolution rate thereof
A technology of vornorazan fumarate and fumaric acid, which is applied in the field of medicine, can solve the problems of high requirements on material fluidity, unfavorable product preparation process, poor powder fluidity, etc. Excellent stability and stable tablet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Tablet formulation (1000 formulation units):
[0050]
[0051]
[0052] * The particle size of the active component after crushing is d90=83.38μm
[0053] The preparation method of the present invention comprises the following steps:
[0054] (1) Pretreatment: the active ingredient is pulverized through a 60-mesh sieve by a universal pulverizer for subsequent use, and other auxiliary materials are sieved for subsequent use;
[0055] (2) Pre-mixing: put the active ingredient, mannitol, fumaric acid, microcrystalline cellulose 102, hydroxypropyl cellulose LXF, and cross-linked carmellose sodium into a hopper mixer (HLS-10, Zhejiang Small Lun) in mixing (20rpm, 10min);
[0056] (3) Dry granulation: put the mixed powder in a dry granulator (GL1-25, created by Zhangjiagang), adjust the roller pressure to 2.0-4.0MPa, the roller speed to 10-15rpm, the cutter speed to 10-15rpm, collect For particles between 30 mesh and 100 mesh (the particles exceeding the upper and l...
Embodiment 2
[0062] Tablet formulation (1000 formulation units):
[0063]
[0064]
[0065] * The particle size of the active component after crushing is d90=63.31μm
[0066] The preparation method of the present invention comprises the following steps:
[0067] (1) Pretreatment: use a universal pulverizer to pulverize the active ingredient through an 80-mesh sieve for subsequent use, and sieve other auxiliary materials for subsequent use;
[0068] (2) Pre-mixing: put the active ingredient, mannitol, fumaric acid, microcrystalline cellulose 102, hydroxypropyl cellulose LXF, and cross-linked carmellose sodium into a hopper mixer (HLS-10, Zhejiang Small Lun) in mixing (20rpm, 10min);
[0069] (3) Dry granulation: put the mixed powder in the dry granulator, adjust the roller pressure to 2.0-4.0MPa, the roller speed to 10-15rpm, the cutter speed to 10-15rpm, and collect the particles between 30 mesh and 100 mesh (the particles exceeding the upper and lower limits are collected by gra...
Embodiment 3
[0075] Tablet formulation (1000 formulation units):
[0076]
[0077]
[0078] * The particle size of the active component after crushing is d90=20.12μm
[0079] The preparation method of the present invention comprises the following steps:
[0080] (1) Pretreatment: the active ingredient is pulverized through a 100-mesh sieve by a universal pulverizer for subsequent use, and other auxiliary materials are sieved for subsequent use;
[0081] (2) Pre-mixing: put the active ingredient, mannitol, fumaric acid, microcrystalline cellulose 102, hydroxypropyl cellulose LXF, and cross-linked carmellose sodium into a hopper mixer (HLS-10, Zhejiang Small Lun) in mixing (20rpm, 10min);
[0082] (3) Dry granulation: put the mixed powder in the dry granulator, adjust the roller pressure to 2.0-4.0MPa, the roller speed to 10-15rpm, the cutter speed to 10-15rpm, and collect the particles between 30 mesh and 100 mesh (the particles exceeding the upper and lower limits are collected b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com