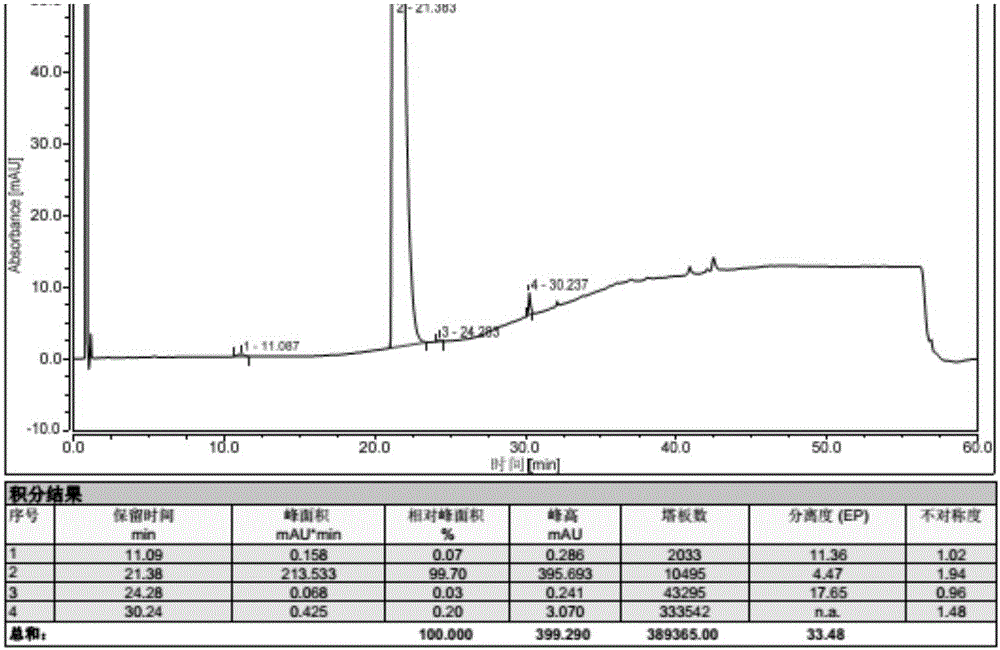
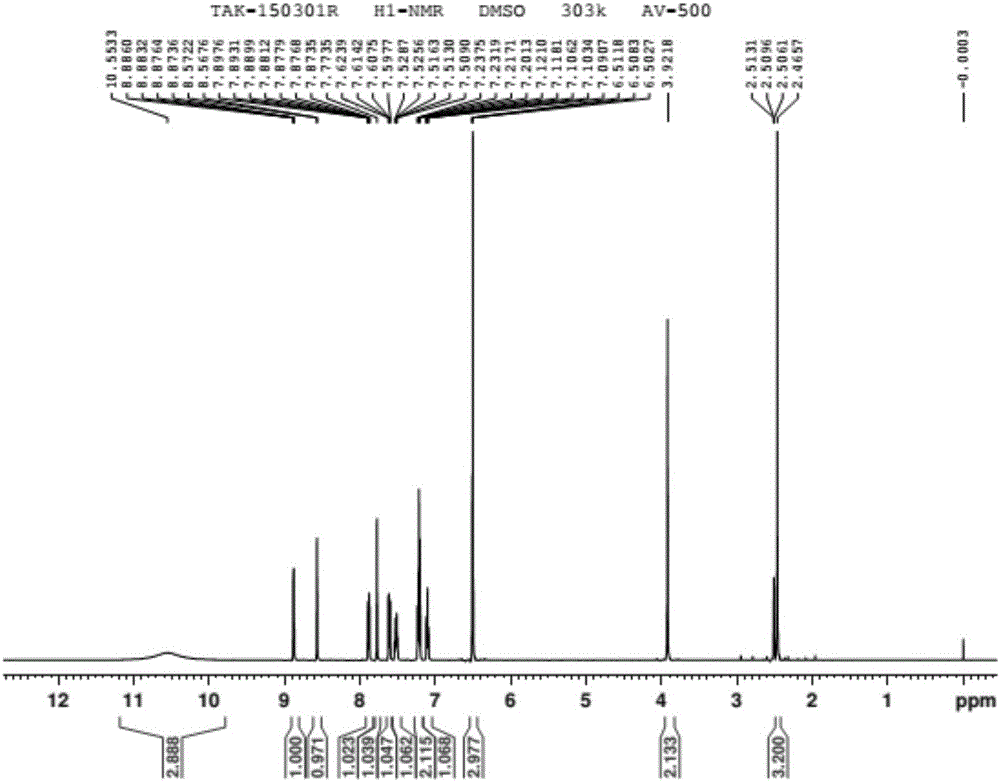
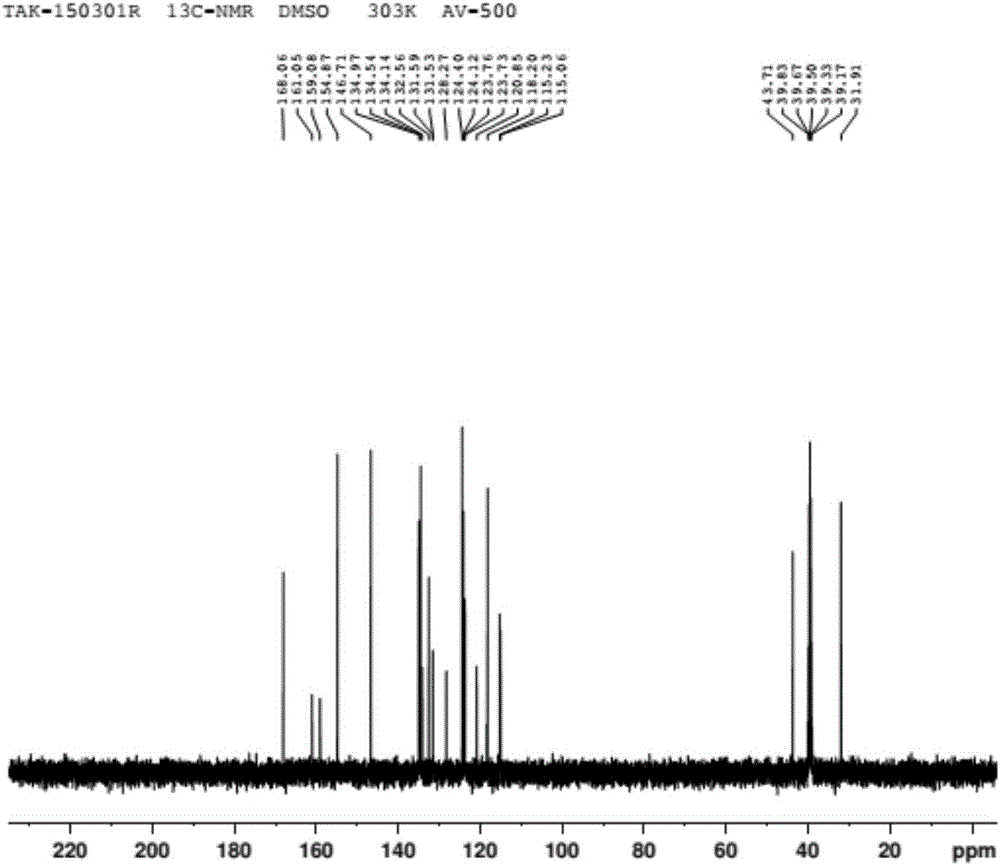
Preparation method of vonoprazan fumarate
A technology of vonoprazan fumarate and methyl carbamic acid, which is applied in the field of medicine, can solve the problems of unfavorable industrial production of vonoprazan fumarate, a total yield of less than 40%, and cumbersome post-processing operations, and achieve The synthesis process is safe and reliable, the equipment is simple, and the effect of post-processing is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] S1: Synthesis of 1-(5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine (II):
[0068]
[0069] 37.8g of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (I) was dissolved in 160ml of methanol at room temperature, and 51.2g of 40% methylamino alcohol solution was added dropwise, and stirred at 25-35°C for 6-8 hours , add 15.1g sodium borohydride in batches under ice bath, react at 20-30°C for 1-2 hours, add 58g of acetone under ice bath to quench, add 200ml ethyl acetate and purified water, extract, add 5% dilute organic phase Adjust the pH to 2-3 with hydrochloric acid, add 20% potassium carbonate to the water phase to adjust the pH to 9-10, add 200ml of ethyl acetate for extraction, wash the organic phase with purified water until neutral, extract once with saturated sodium chloride solution, anhydrous sulfuric acid After drying over sodium, the solvent was removed to obtain 38.0 g of 1-(5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine as a white solid crude p...
Embodiment 2
[0085] S1: Synthesis of 1-(5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine (II):
[0086] 47.2g of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (I) was dissolved in 200ml of methanol at room temperature, 64g of 40% methylamino alcohol solution was added dropwise, stirred at 25-35°C for 6-8 hours, Add 33.7g potassium borohydride in batches under ice bath, react at 20-30°C for 1-2 hours, add 72.5g acetone under ice bath to quench, add 200ml ethyl acetate and purified water, extract, add 5% dilute organic phase Adjust the pH to 2-3 with hydrochloric acid, add 20% potassium carbonate to the water phase to adjust the pH to 9-10, add 200ml of ethyl acetate for extraction, wash the organic phase with purified water until neutral, extract once with saturated sodium chloride solution, anhydrous sulfuric acid After drying over sodium, the solvent was removed to obtain 46.5 g of crude 1-(5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine (II) as a white solid, with a yield of ...
Embodiment 3
[0096] S1: Synthesis of 1-(5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine (II):
[0097] 47.25g of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (I) was dissolved in 200ml of methanol at room temperature, 64g of 40% methylamino alcohol solution was added dropwise, stirred at 25-35°C for 6-8 hours, Add 13.6g of lithium borohydride in batches under ice bath, react at 20-30°C for 1-2 hours, add 72.5g of acetone under ice bath to quench, add 200ml of ethyl acetate and purified water, extract, add 5% dilute organic phase Adjust the pH to 2-3 with hydrochloric acid, add 20% potassium carbonate to the water phase to adjust the pH to 9-10, add 200ml of ethyl acetate for extraction, wash the organic phase with purified water until neutral, extract once with saturated sodium chloride solution, anhydrous sulfuric acid After sodium drying, the solvent was removed to obtain 45.45 g of 1-(5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methylmethylamine (II) as a white solid crude product, with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com