Preparation method of vonoprazan fumarate
A technology of vonoprazan fumarate and pyridine, which is applied in the field of preparation of vonoprazan fumarate, and can solve problems such as potential safety hazards, long routes, low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
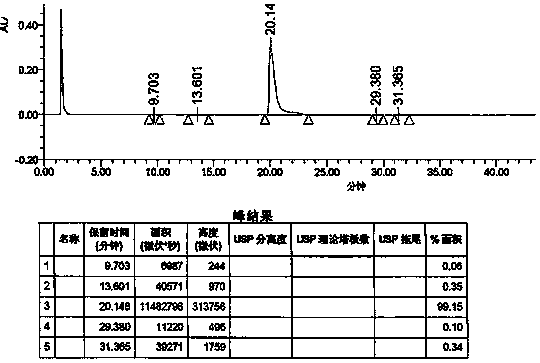
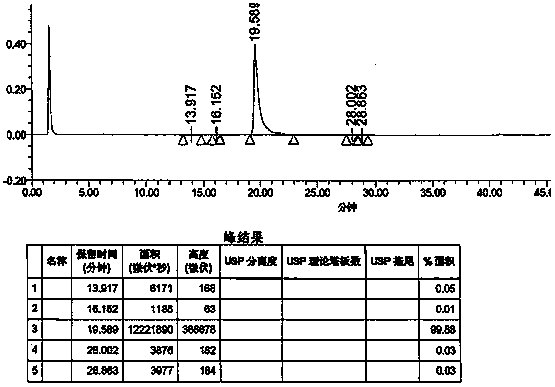
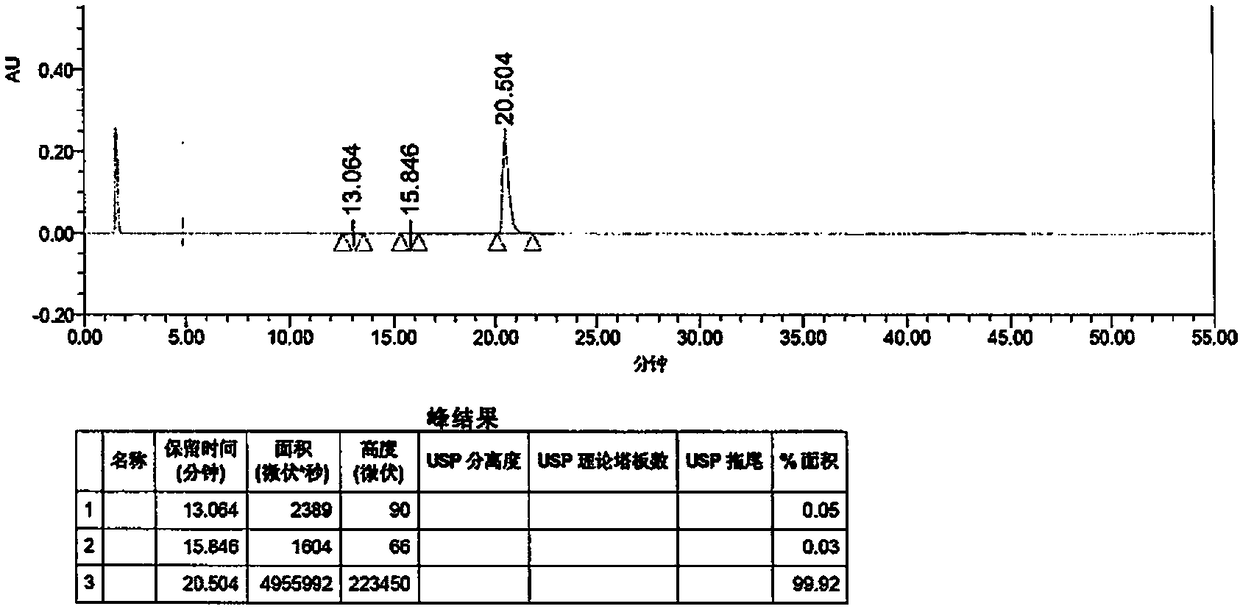
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Formula (5) 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbonitrile
[0040] 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (100g, 537.08mmole), 4-dimethylaminopyridine (9.84g, 80.56mmole), diisopropylethylamine (97.18g, 751.81mmole), acetonitrile (600ml) was added into a three-necked flask (3000ml), stirred and dissolved at 10-20°C, and then added dropwise to pyridine-3-sulfonyl chloride (114.47g, 644.5mmole) in acetonitrile (200ml) Solution, control the internal temperature of the reaction solution to not exceed 30°C, and stir at this temperature for 3 hours after the dropwise addition is completed. Then add 1N hydrochloric acid to adjust the pH value to 4-5, then add 1200ml of water, mechanically stir to crystallize, filter, and dry the solid. Add the solid to 1000ml of acetonitrile, heat at 60-70°C to dissolve, add activated carbon to decolorize, filter, mechanically stir the filtrate, add 1000ml of water drop by drop, stir and...
Embodiment 2
[0041]Example 2: Preparation of Formula (5) 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbonitrile
[0042] 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (100g, 537.08mmole), 4-dimethylaminopyridine (10.82g, 88.616mmole), diisopropylethylamine (92.32g, 714.21mmole), acetonitrile (700ml) was added to a three-necked flask (3000ml), stirred and dissolved at 10-20°C, and then added dropwise to pyridine-3-sulfonyl chloride (120.19g, 676.73mmole) in acetonitrile (300ml) Solution, control the internal temperature of the reaction solution to not exceed 30°C, and stir at this temperature for 3.5 hours after the dropwise addition is completed. Then add 1N hydrochloric acid to adjust the pH value to 4-5, then add 1100ml of water, mechanically stir and crystallize, filter, and dry the solid. Add the solid to 1100ml of acetonitrile, heat to 60-70°C to dissolve, add activated carbon to decolorize, filter, mechanically stir the filtrate, add 1100ml of water drop by drop, stir ...
Embodiment 3
[0043] Example 3: Preparation of Formula (4) 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde
[0044] 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbonitrile (150g, 458.2mmole), tetrahydrofuran (600ml), acetic acid (390g), water (600ml ), pyridine (750g) into a 5L three-necked flask, stirred to dissolve, then added Raney nickel (wet weight 75g), the reaction temperature was 15-25°C, stirred and added sodium hypophosphite (300g). After addition, react for 4 hours, filter, separate layers, the lower layer is the water layer, discard, the upper liquid is the organic layer, and concentrate under reduced pressure at 80°C to about 600ml, add 600ml of water and 600ml of ethyl acetate to the concentrated solution, and separate the organic layer. layer, and the aqueous layer was extracted with 240 ml of ethyl acetate, and the combined organic layers were concentrated under reduced pressure at 80°C. When the liquid cannot be concentrated, add 240ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com