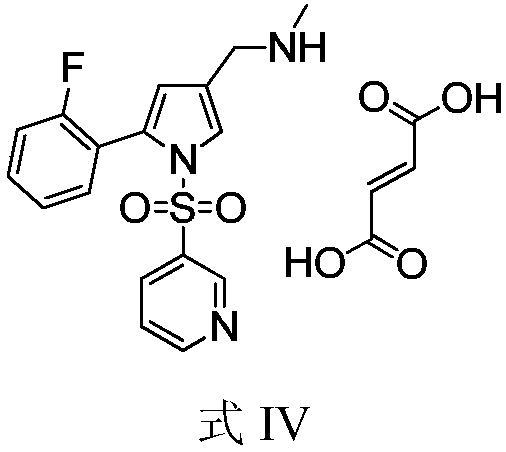
New preparation method of Vonoprazan
A technology of amides and compounds, applied in the new field of preparation, can solve the problems of many operation steps, expensive, ultra-low temperature for reaction, etc., and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1.5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carboxylic acid ethyl ester
[0077]
[0078] To a solution of ethyl 5-(2-fluorophenyl)-1H-pyrrole-3-carboxylate (4 g) in tetrahydrofuran (50 mL) was added sodium hydride (60% in oil, 0.5 g), and the mixture was Stir for 30+ minutes. Pyridine-3-sulfonyl chloride hydrochloride (1 g) was added, followed by stirring for more than 3 hours. After the reaction was completed, it was quenched with saturated brine, and the mixture was extracted with 50 mL of ethyl acetate. The extract was washed with saturated brine and concentrated. Add methyl tert-butyl ether and ethyl acetate (1:1) for crystallization, and obtain colorless crystals (yield 80%, purity 96%).
[0079] l H-NMR (CDC1 3 )δ: 1.35 (3H, t, J = 7.2Hz), 4.30 (2H, q, J = 7.2Hz), 6.69 (1H, d, J = 1.8Hz), 6.99-7.09 (lH, m), 7.19- 7.19(2H,m),7.40-7.39(1H,m),7.46-7.52(1H,m),7.61-7.75(1H,m),8.15(1H.d,J=1.8Hz),8.59-8.61( lH, m), 8.82-8.84 (lH, m).
Embodiment 2
[0080] Example 2.5-(2-fluorophenyl)-N-methyl 1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carboxamide
[0081]
[0082] Add 37g of ethyl 5-(2-fluorophenyl)-1H-pyrrole-3-carboxylate into 400mL of water, add dropwise 10mL of 40% methylamine aqueous solution, raise the temperature to about 75°C, and react for more than 4 hours. After the reaction, cool down to 0-10° C., stir for more than 12 hours to precipitate an off-white solid, filter, and dry to obtain 33 g of the product, with a yield of 94% and a purity of 95%. l H-NMR (CDC1 3 )δ: 2.65(3H), 6.33(1H, d, J=1.8Hz), 7.28(2H, m), 7.49-7.75(4H, m), 8.15(1H.d, J=1.8Hz), 8.42- 8.45(2H,m), 8.91(lH,s).
Embodiment 3
[0083] Example 3.5-(2-fluorophenyl)-N-methyl 1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carboxamide
[0084]
[0085] Add 4 g of ethyl 5-(2-fluorophenyl)-1H-pyrrole-3-carboxylate into 40 mL of water, add dropwise 1 mL of 40% methylamine aqueous solution, heat up to about 55°C, and react for more than 4 hours. After the reaction, cool down to 0-10°C, stir for more than 12 hours to precipitate an off-white solid, filter, and dry to obtain 3 g of the product with a yield of 79% and a purity of 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com