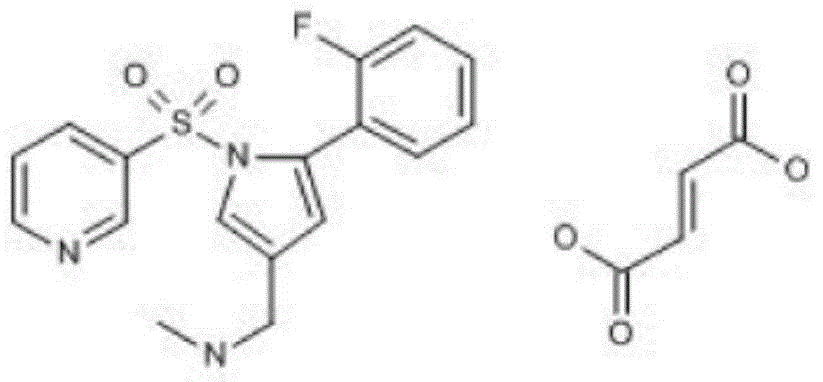
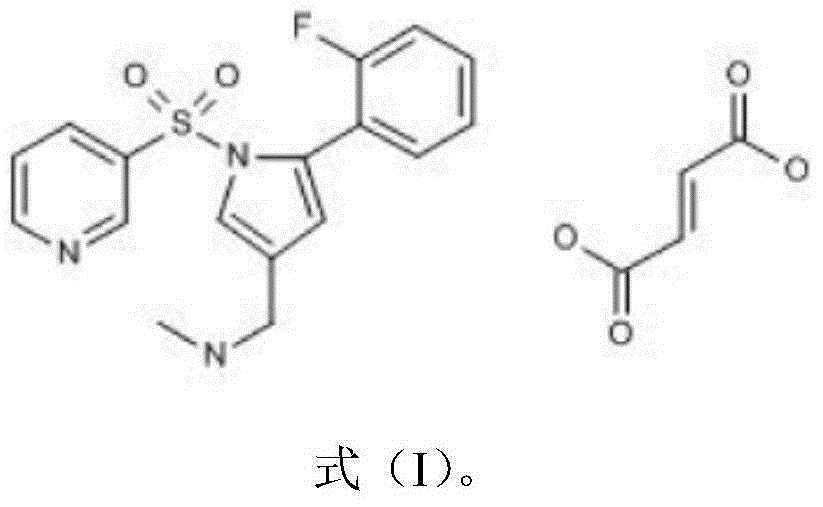
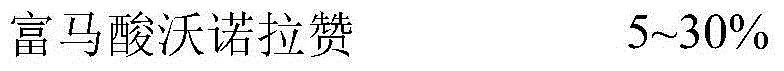Pharmaceutical composition with vonoprazan fumarate and preparation method thereof
A composition and drug technology, applied in the field of stable pharmaceutical compositions, can solve the problems of reducing the acidity of pharmaceutical compositions, and achieve the effect of superior storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1 (sample 5)
[0086] [Table 9] Chip composition
[0087]
[0088]
[0089] ▲When necessary, use microcrystalline cellulose as an adjustment component to change the content.
[0090] [Table 10] Composition of film coating aqueous solution
[0091]
[0092] ▲Purified water accounts for 90% (w / w) of the film coating aqueous solution.
[0093] A plain tablet (core tablet) containing voronoprazan fumarate was prepared according to the composition ratio shown in the above table [9].
[0094] Dissolve 2.6 g of hydroxypropyl cellulose in 87.9 g of purified water to obtain a binder solution. Add the prepared binder solution to the excipients consisting of vonoprazan fumarate (26.72g), mannitol (130.98g) and microcrystalline cellulose (46.5g) for wet granulation. After drying, the granules were sized with a 30-mesh sieve, and magnesium stearate (2.2 g) and croscarmellose sodium (11 g) were added for total mixing. Then the obtained granules are pressed in...
Embodiment 2
[0096] Embodiment 2 (sample 6)
[0097] [Table 11] Chip composition
[0098]
[0099]
[0100] ▲When necessary, use microcrystalline cellulose as an adjustment component to change the content.
[0101] [Table 12] Composition of film coating aqueous solution
[0102]
[0103] ▲Purified water accounts for 90% (w / w) of the film coating aqueous solution.
[0104] A plain tablet (core tablet) containing vonoprazan fumarate was prepared according to the composition ratio shown in the above table [11].
[0105] Dissolve 1.3 g of hydroxypropyl cellulose and 0.75 g of fumaric acid in 43.95 g of purified water to obtain a binder solution. The prepared binder solution was added to an excipient composed of vonoprazan fumarate (13.36g), mannitol (57.99g) and microcrystalline cellulose (30g) for wet granulation. After drying, the granules were sized with a 30-mesh sieve, and magnesium stearate (1.1 g) and croscarmellose sodium (5.5 g) were added for total mixing. Then the obta...
Embodiment 3
[0107] Embodiment 3 (sample 7)
[0108] [Table 13] Chip composition
[0109]
[0110]
[0111] ▲When necessary, use microcrystalline cellulose as an adjustment component to change the content.
[0112] [Table 14] Composition of film coating aqueous solution
[0113]
[0114] ▲Purified water accounts for 90% (w / w) of the film coating aqueous solution.
[0115] A plain tablet (core tablet) containing vonoprazan fumarate was prepared according to the composition ratio shown in the above table [13].
[0116] Dissolve 3.3 g of hydroxypropyl cellulose in 43.95 g of purified water to obtain a binder solution. The prepared binder solution was added to the excipients consisting of vonoprazan fumarate (13.36g), mannitol (70.99g) and microcrystalline cellulose (15.75g) for wet granulation. After drying, the granules were sized with a 30-mesh sieve, and magnesium stearate (1.1 g) and croscarmellose sodium (5.5 g) were added for total mixing. Then the obtained granules are pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com