Preparation method of low-impurity Vonoprazan fumarate
A technology of vonorazan fumarate and impurities, applied in the field of medicine, can solve the problems of difficult realization, unrealistic feeding weight, low yield, etc., and achieve the effects of prolonging the process cycle, saving energy, and reducing the introduction of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Voronolazan was prepared with reference to Comparative Example 7, HPLC purity 94.45%, impurity content: the sum of impurity A and impurity C was 0.71%, impurity B was not detected, impurity D was 0.31%, and impurity E was 0.14%. Add 15.0 kg of ethanol to the residue, add 1.53 kg of 48% hydrobromic acid dropwise at room temperature, after the dropwise addition, stir at room temperature for 1 h, filter, rinse the filter cake with 9.0 kg of ethanol, and dry the filter cake to obtain Vonuolazan hydrobromide. 2.95kg, the yield was 76.22%. HPLC purity 98.38%, impurity content: impurity A is 0.03%, impurity B is 0.01%, impurity C is 0.01%, impurity D is 0.01%, and impurity E is 0.13%.
[0090] Add 12.5 kg of ethyl acetate, 1.65 kg of ammonia, 12.5 kg of purified water, and 2.5 kg of voronolazan hydrobromide to a 50L reaction kettle. After stirring until the system is free of solids, continue to stir for 15 minutes, stand for phase separation, and add 6.25 to the water phase. kg ...
Embodiment 2
[0093] Voronolazan was prepared with reference to Comparative Example 7, HPLC purity 94.54%, impurity content: the sum of impurity A and impurity C was 0.61%, impurity B was not detected, impurity D was 0.22%, and impurity E was 0.12%. Add 15.0 kg of ethanol to the residue, add 1.53 kg of 48% hydrobromic acid dropwise at room temperature, after the dropwise addition, stir at room temperature for 1 h, filter, rinse the filter cake with 9.0 kg of ethanol, and dry the filter cake to obtain Vonuolazan hydrobromide. 2.98kg, the yield was 76.99%. HPLC purity 98.03%, impurity content: impurity A is 0.11%, impurity B is not detected, impurity C is not detected, impurity D is not detected, impurity E is 0.06%.
[0094] Add 12.5 kg of ethyl acetate, 1.65 kg of ammonia, 12.5 kg of purified water, and 2.5 kg of voronolazan hydrobromide to a 50L reaction kettle. After stirring until the system is free of solids, continue to stir for 15 minutes, stand for phase separation, and add 6.25 to the ...
Embodiment 3
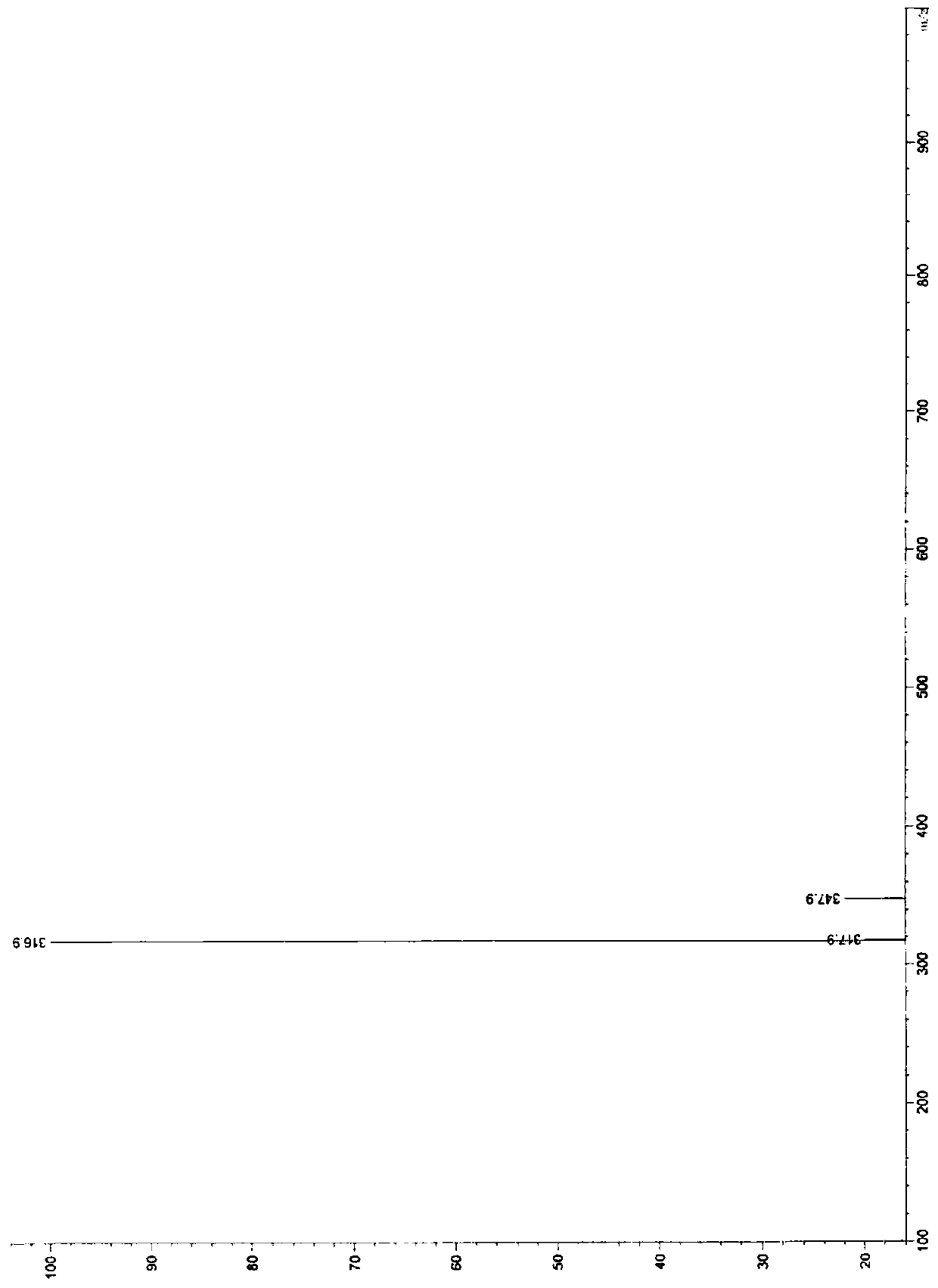
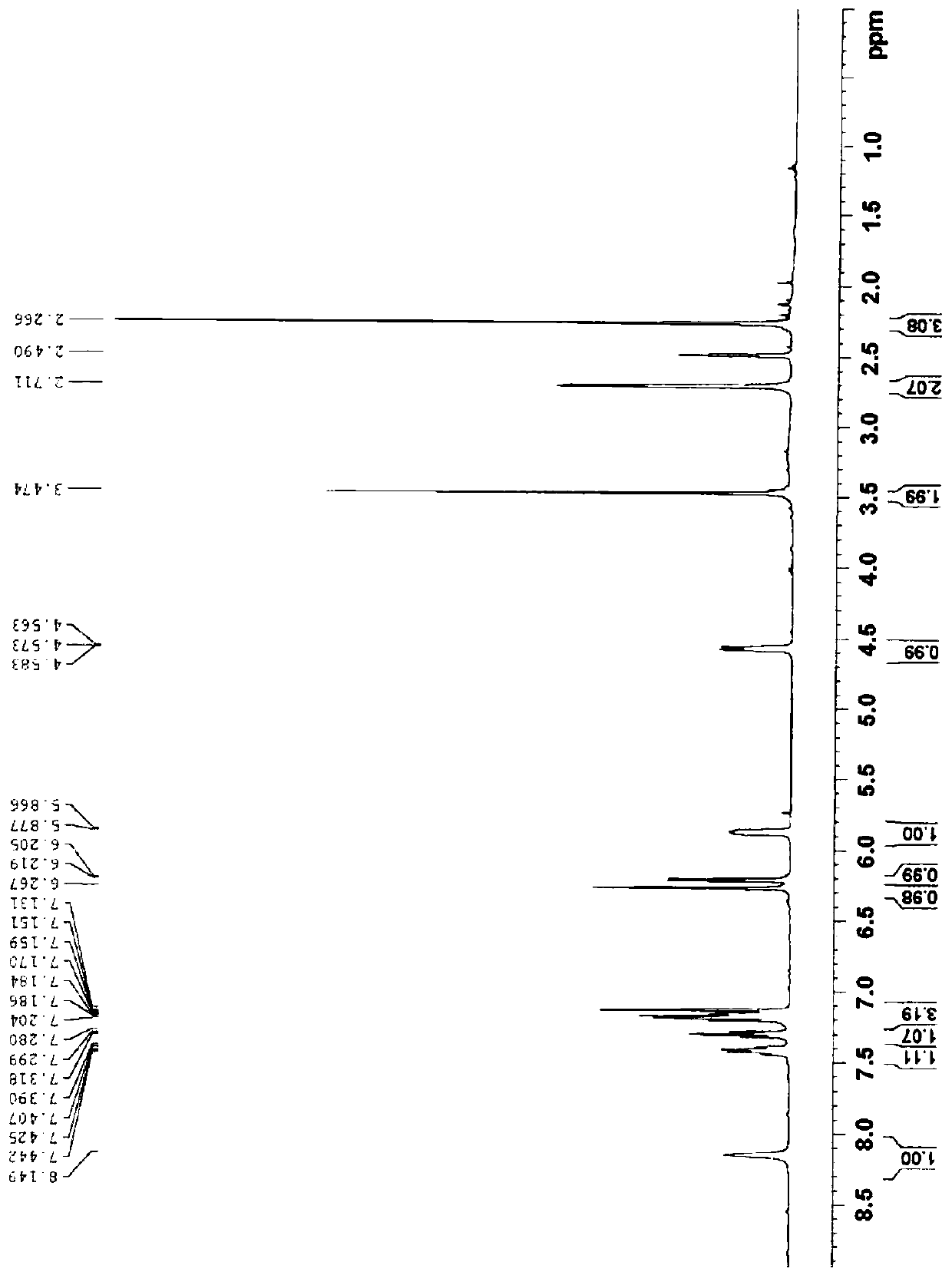
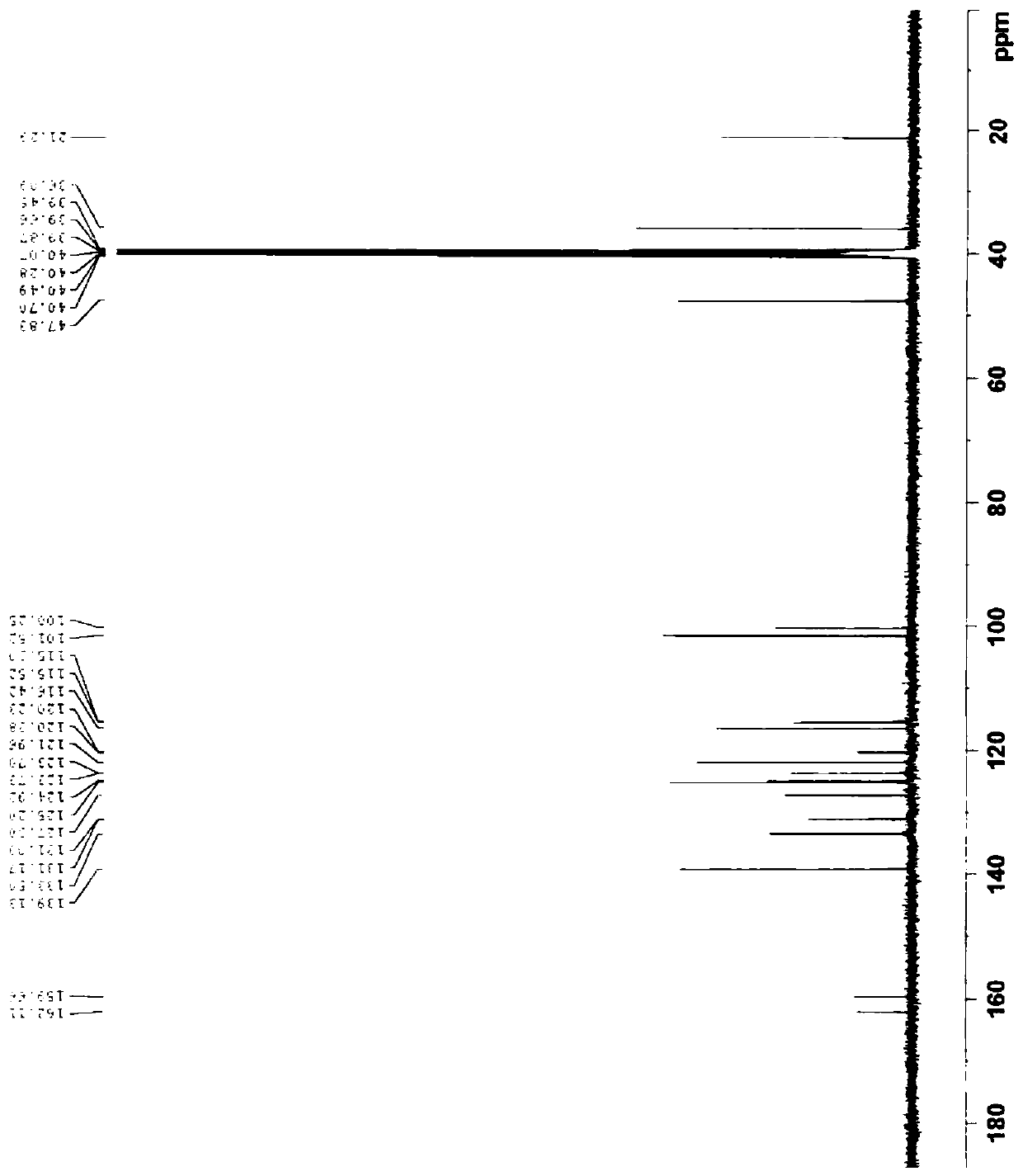
[0103] Example 3 Preparation of impurity D. 15.0g of Voronolazan was dissolved in 50mL of methanol, and 15.0g of sodium borohydride was added dropwise to 100mL of N,N-dimethylacetamide solution under stirring. After the addition was completed, stirred at room temperature for 12h, and 1mL was taken to react. Add 5ml of purified water and 1ml of ethyl acetate, shake well, let stand, take the organic phase, HPLC detection, the organic phase appears 4 large over-reduced impurity peaks, followed by impurity B (relative retention time RTT=0.28), impurity C (RTT=0.52), impurity A (RTT=0.54), impurity D (RTT=0.73), add 500 mL of purified water and 250 mL of ethyl acetate to the reaction system, stir and stand for phase separation, and concentrate the organic phase under reduced pressure to obtain An oily substance was prepared and separated to obtain 0.90 g of impurity D. Purity 95.06%, MS (ESI) m / z (M+H) + :347.9; 1 H NMR(400MHz, DMSO-d 6 ) δ8.15(s,1H),7.43(dd,J=1.0Hz,6.8Hz,1H), 7.30(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com