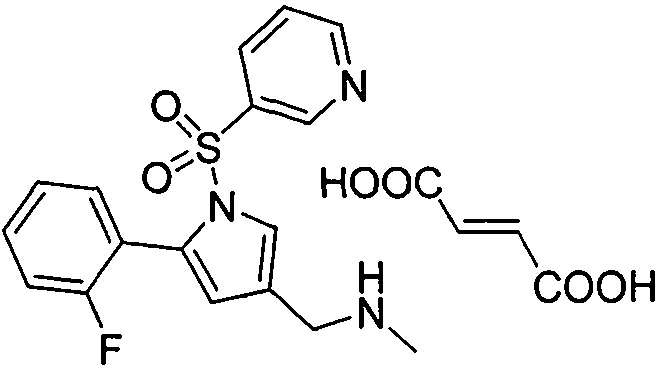
Preparation method of vonoprazan fumarate
A technology of vonoprazan fumarate and Schiff base, which is applied in the field of compound preparation, can solve the problems of low yield and unfavorable industrial scale-up production, and achieve mild reaction treatment conditions, high product purity, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
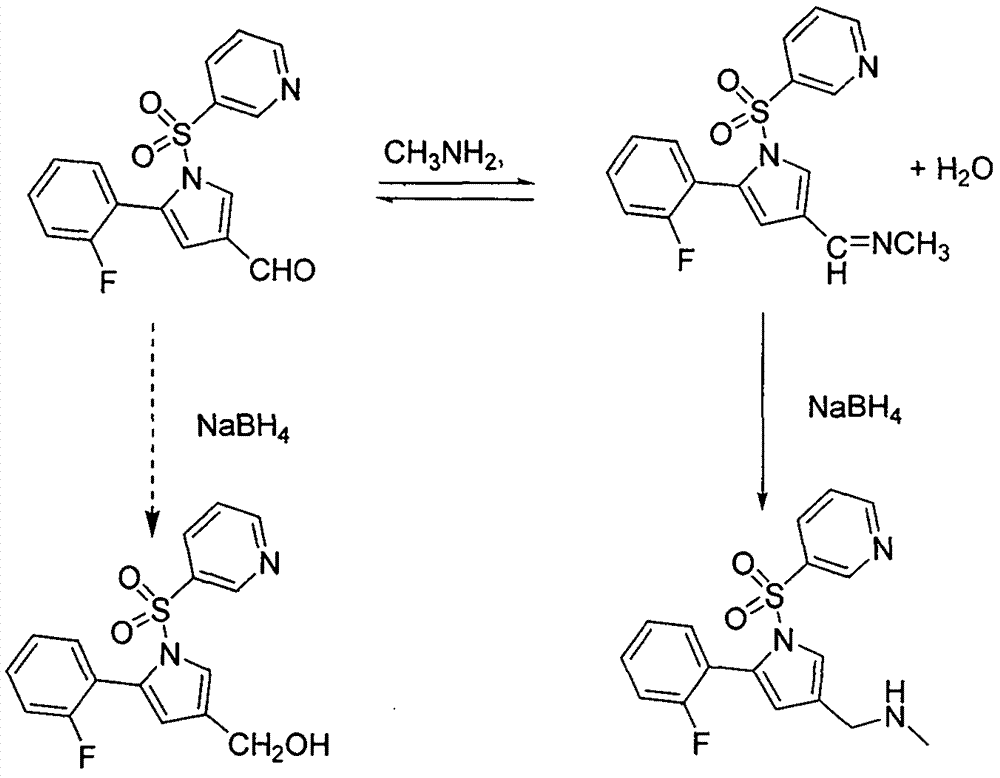
[0021] Add 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1-H-pyrrole-3-carbaldehyde (3.3Kg 10mol) to 15.5 liters of methanol, and add 33 % methylamine methanol solution (4.70Kg 50mol), and maintain the reaction at about 10°C for 6h. Add sodium cyanoborohydride (502 g 8 mol) at 10°C, react for 4 h, TLC to 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1-H-pyrrole-3-carbaldehyde The basic reaction is complete, add 4mol / L hydrochloric acid to adjust PH=7~8, concentrate to dry methanol at 45°C, add 3 liters*2 dichloromethane for extraction, combine the organic phases, dry, filter, add fumaric acid (1160 grams 10mol ), heated to reflux for 2 hours, cooled to room temperature to precipitate solids, filtered, and dried solids weighed 3.91Kg, yield: 85%, HPLC: 98.3%.
Embodiment 2
[0023] Add 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1-H-pyrrole-3-carbaldehyde (3.3Kg 10mol) to 15.5 liters of methanol, and add 33 % methylamine methanol solution (4.70Kg 50mol), and maintain the reaction at about 10°C for 6h. Add sodium triacetoxyborohydride (2.54Kg 12mol) at 0°C, react for 6 hours, TLC until the raw materials are basically reacted completely, add 4mol / L sodium hydroxide to adjust pH=7~8, concentrate to dry methanol at 45°C, add 3 liter*2 dichloromethane extraction, combined organic phases, dried, filtered, added fumaric acid (1160 g 10 mol) to the filtrate, heated to reflux for 2 h, cooled to room temperature to precipitate solids, filtered, and the dried solids weighed 4.38 Kg. Yield: 95%, HPLC: 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com