Method for synthesizing and refining cinacalcet hydrochlorid
A technology of cinacalcet hydrochloride and refining method, which is applied in the field of synthesis and refining of cinacalcet hydrochloride bulk drug, can solve the problems of small reaction scale, insufficient purity of raw material drug, difficult purification, etc., and achieve short reaction steps and raw material The effect of convenient source and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
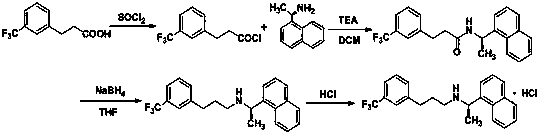
[0042] The present invention will be further described below by specific embodiment.
[0043] The first step: the synthesis of 3-(trifluoromethyl) phenylpropionyl chloride:
[0044] Weigh 3 - Add 20.0 g of (trifluoromethyl)phenylpropionic acid to 100.0 ml of toluene, stir evenly, slowly add 40.0 ml of thionyl chloride to the reaction system, slowly raise the temperature to 80 °C and stir for 3 h, after the reaction, remove the residual under reduced pressure Thionyl chloride and solvent, get 3 - (Trifluoromethyl)phenylpropionyl chloride.
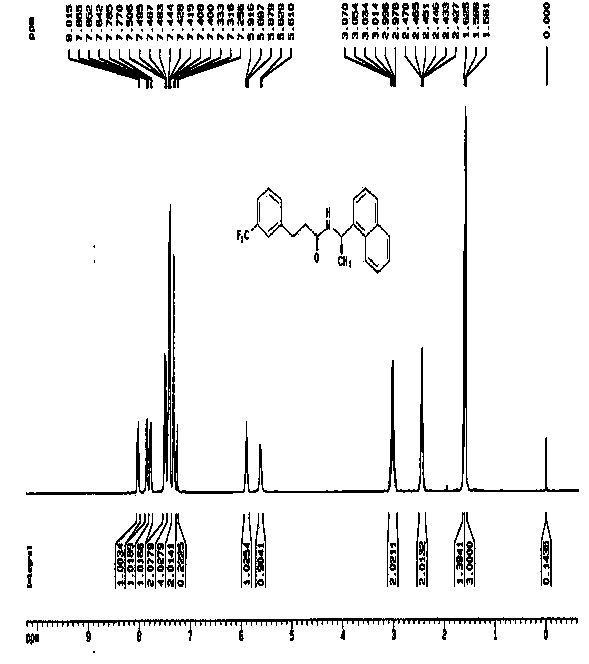
[0045] The second step: the synthesis of N-((1R)-1-(1-naphthyl)ethyl)-3-(3-(trifluoromethyl)phenyl)propanamide:
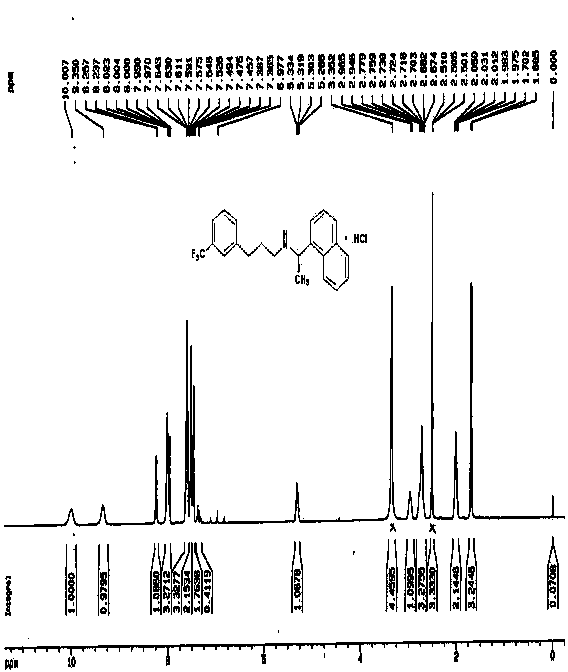
[0046] Weigh 15.70g R-1-(1-naphthyl)ethylamine and 13.91g triethylamine, dissolve in 150 ml dichloromethane, dissolve the acid chloride obtained in the previous step reaction in 50 ml dichloromethane, drop slowly into the reaction system, control the reaction temperature at 0 to 20°C, and complete the reaction in 30 min, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com