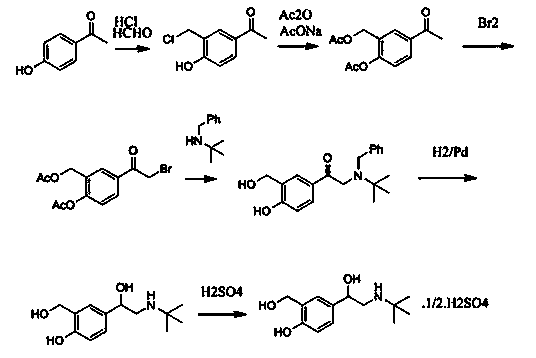
Production technology for synthetizing salbutamol sulphate
A salbutamol sulfate and production process technology, which is applied in the field of synthesizing salbutamol sulfate, can solve the problems of troublesome post-processing, low yield, and many side reactions, and achieve the effects of short reaction steps, easy availability of raw materials, and reduced product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
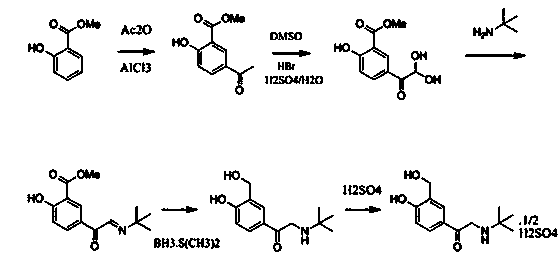
[0043] (1) Preparation of compound 3 (5-bromoacetyl-2-hydroxybenzaldehyde):
[0044] Add 55 grams of anhydrous aluminum chloride to the reaction bottle, stir and add 50 milliliters of anhydrous dichloromethane, 20 milliliters of anhydrous dichloroethane, heat up to 50 ° C, start to drop 23.3 grams of chloroacetyl chloride, dropwise, Start to add 11 grams of salicylaldehyde dropwise. After the dropwise addition, keep the temperature at 50° C. for 20 hours to react. The reaction solution is lowered to room temperature, and slowly added dropwise to the mixed solution of 150 grams of water and 50 milliliters of dichloromethane. Add 20 ml of concentrated hydrochloric acid, stir, separate the organic phase, extract the aqueous phase with 300 ml of dichloromethane × 2, concentrate under reduced pressure at 40-45°C to obtain a yellow solid and a small amount of oil, stir, crystallize and filter under ice-water bath cooling to obtain The light yellow solid was dried to constant weight ...
Embodiment 2
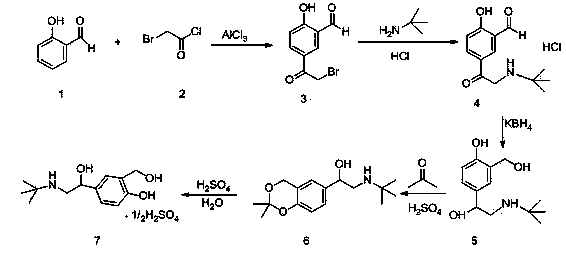
[0056] (1) Preparation of Compound 3:
[0057] Add 110 grams of anhydrous aluminum chloride to the reaction flask, stir and add 100 milliliters of anhydrous dichloromethane, 50 milliliters of anhydrous dichloroethane, heat up to 50 ° C, start to drop 23.3 grams of bromoacetyl chloride, dropwise, Start to add 22 grams of salicylaldehyde dropwise. After the dropwise addition, keep the temperature at 50° C. for 20 hours to react. The reaction solution is lowered to room temperature, and slowly added dropwise to the mixed solution of 300 grams of water and 100 milliliters of dichloromethane. Add 50 ml of concentrated hydrochloric acid, stir, separate the organic phase, extract the aqueous phase with 500 ml of dichloromethane × 2, concentrate under reduced pressure at 40-45°C to obtain a yellow solid and a small amount of oil, stir, crystallize and filter under ice-water bath cooling to obtain Light yellow solid, dried to constant weight to obtain 35.5 g of compound 3.
[0058] (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com