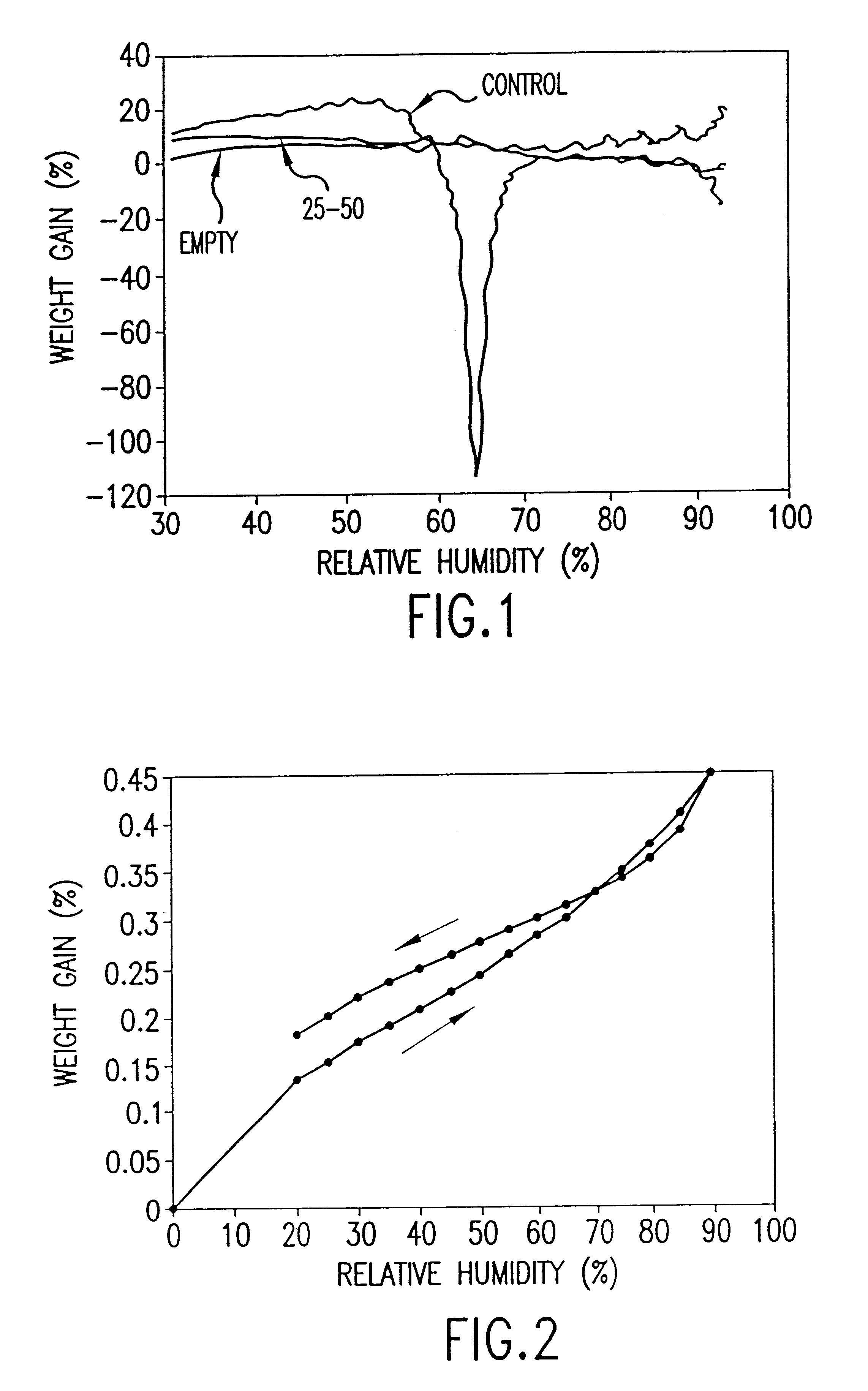
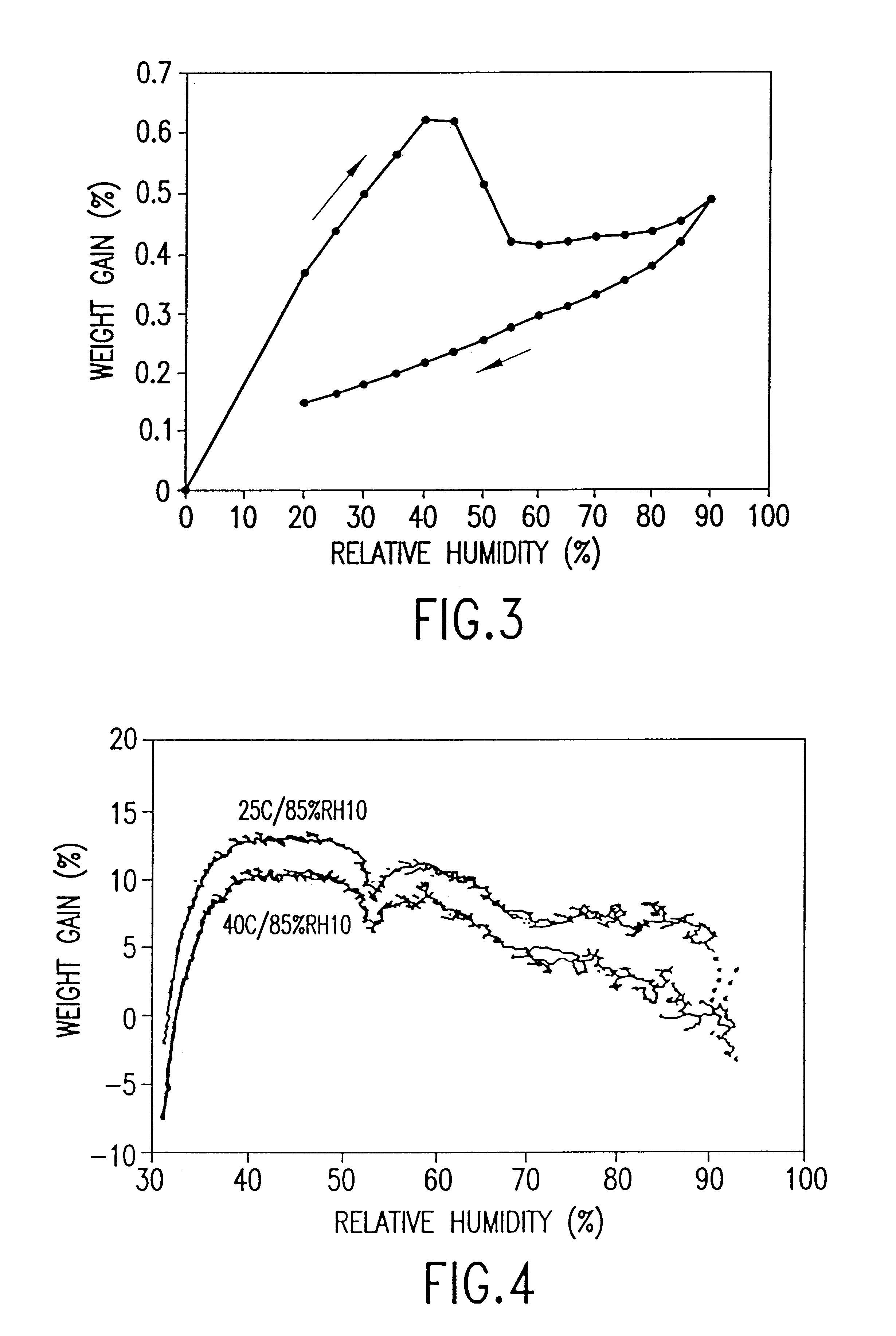
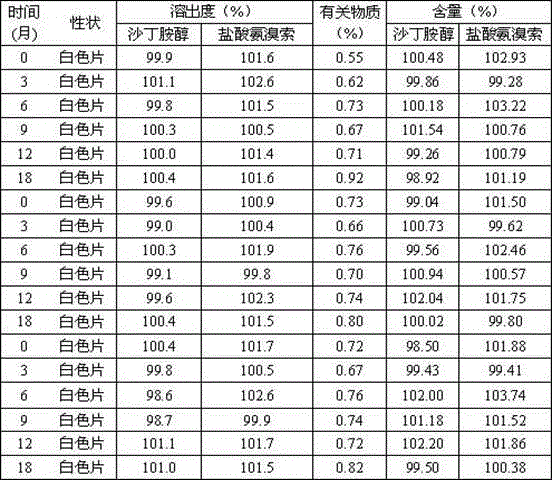
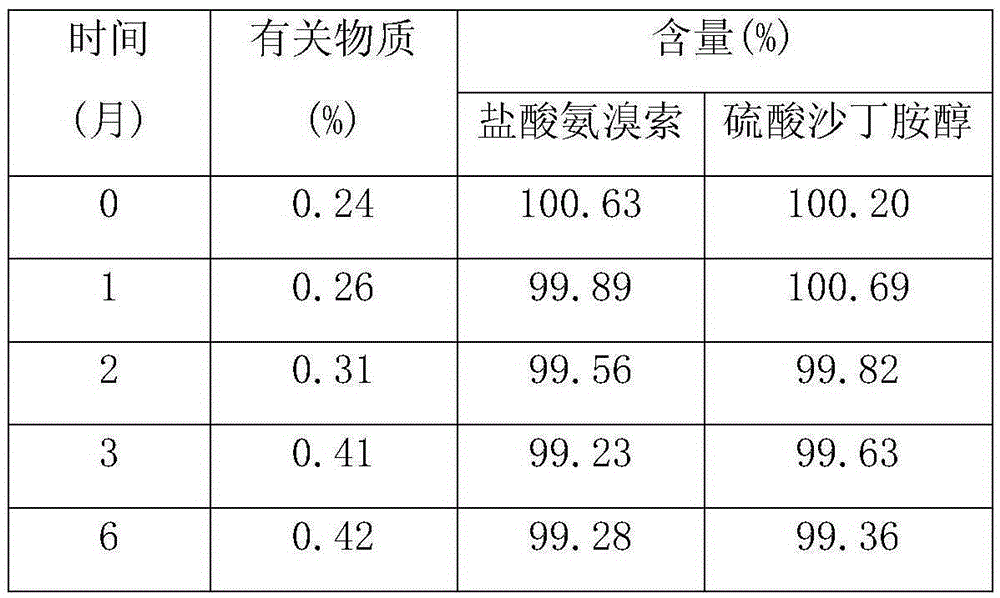
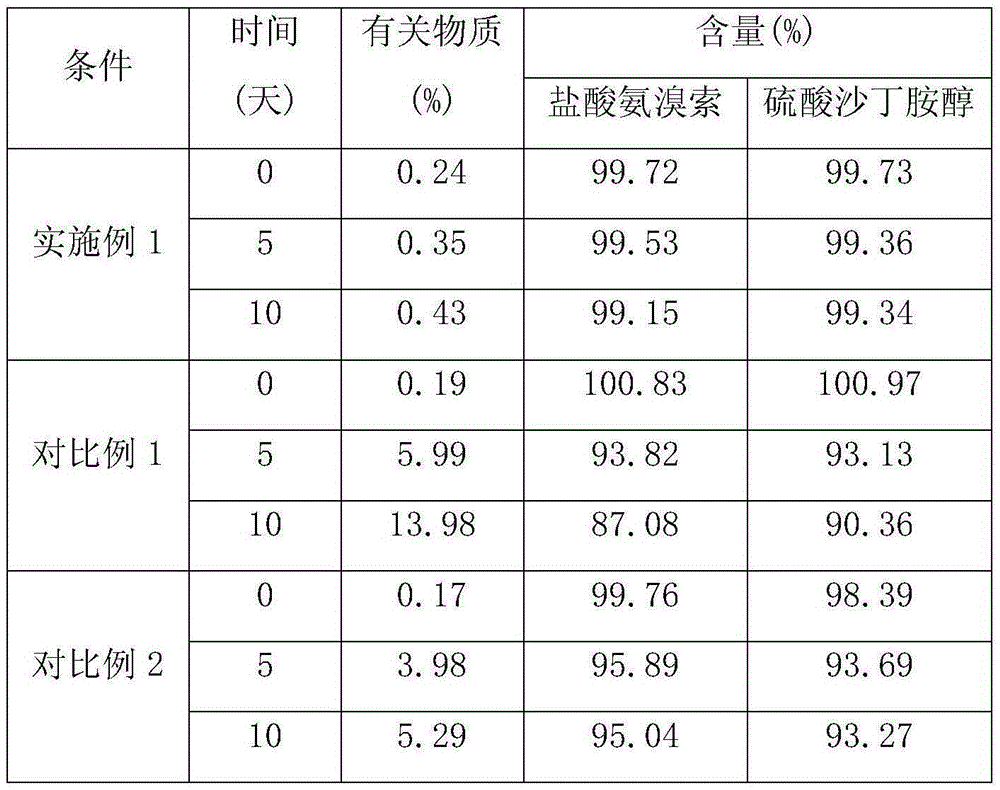
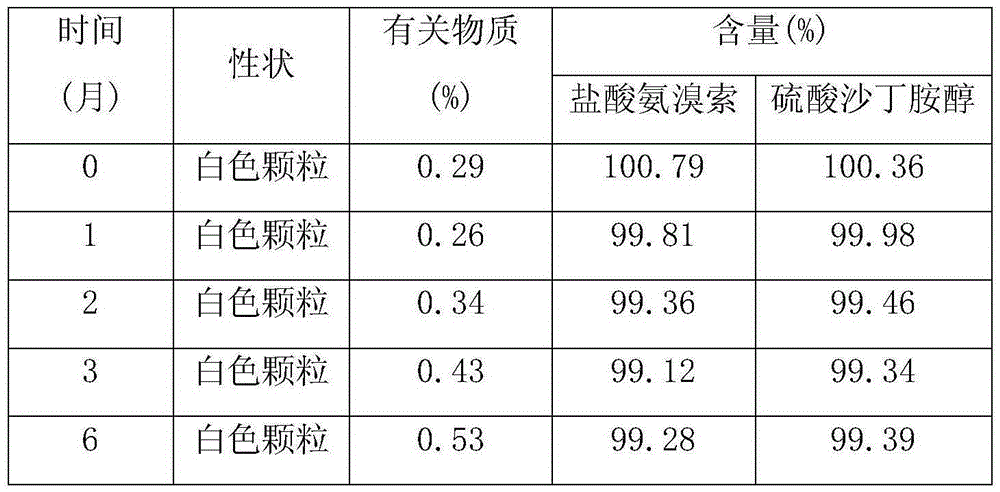
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Salbutamol Sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
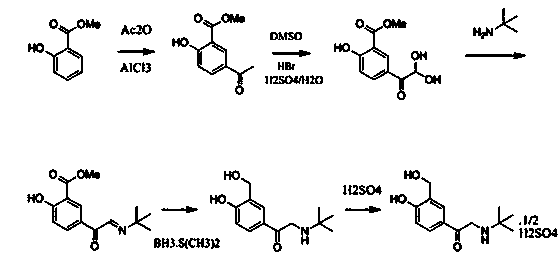
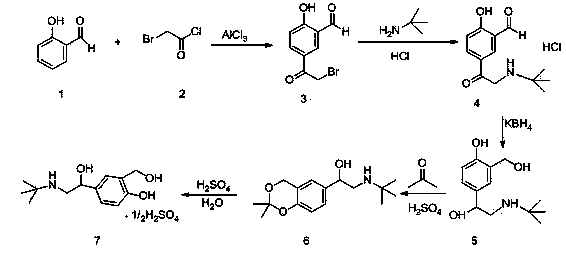
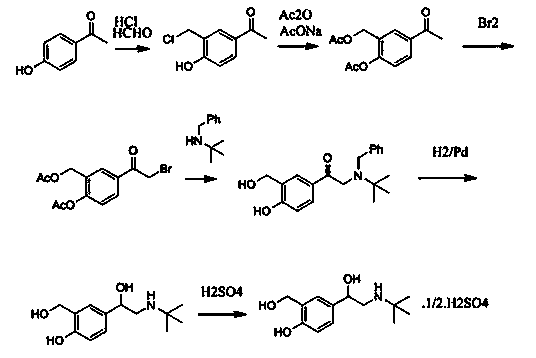
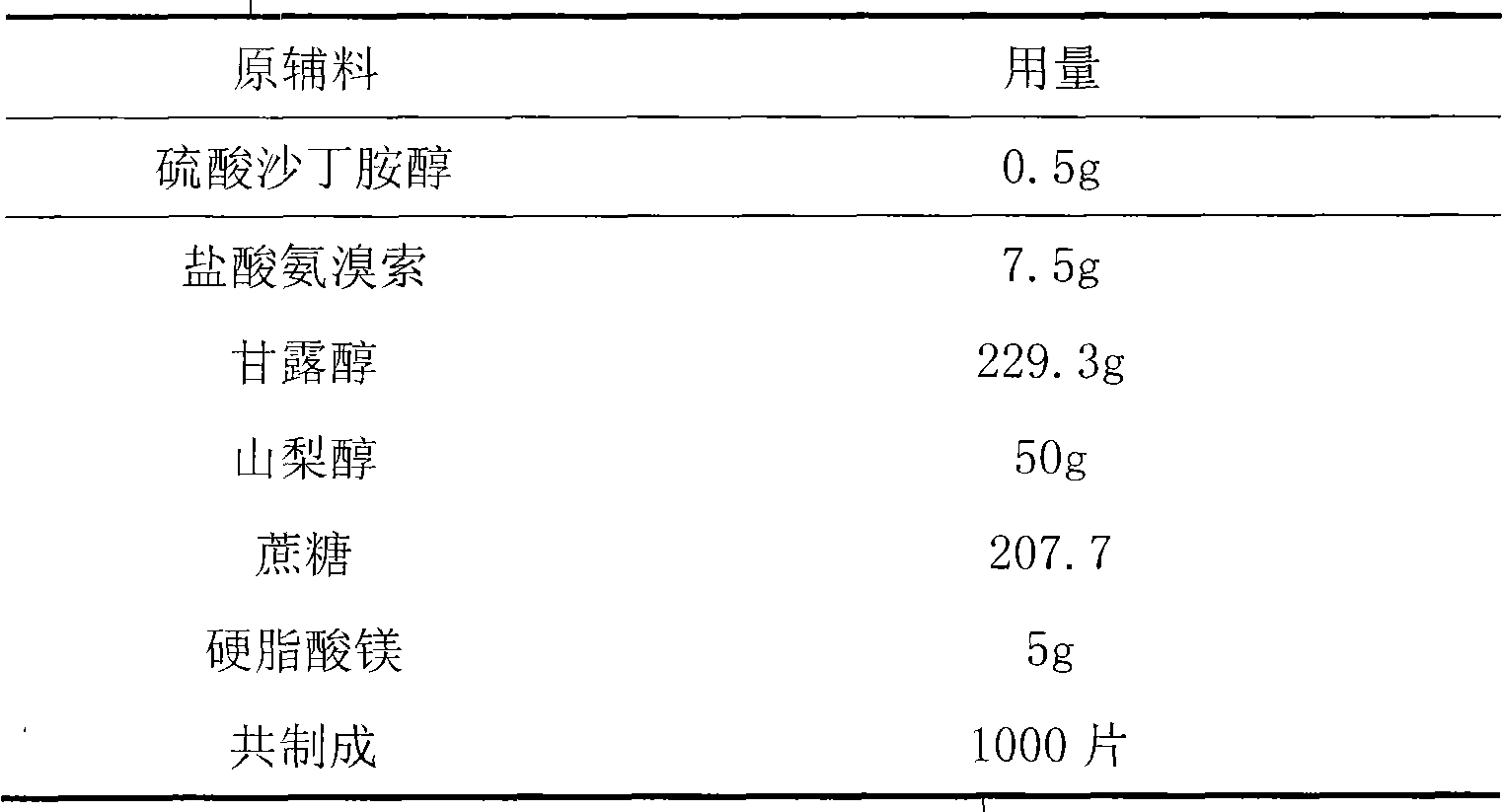
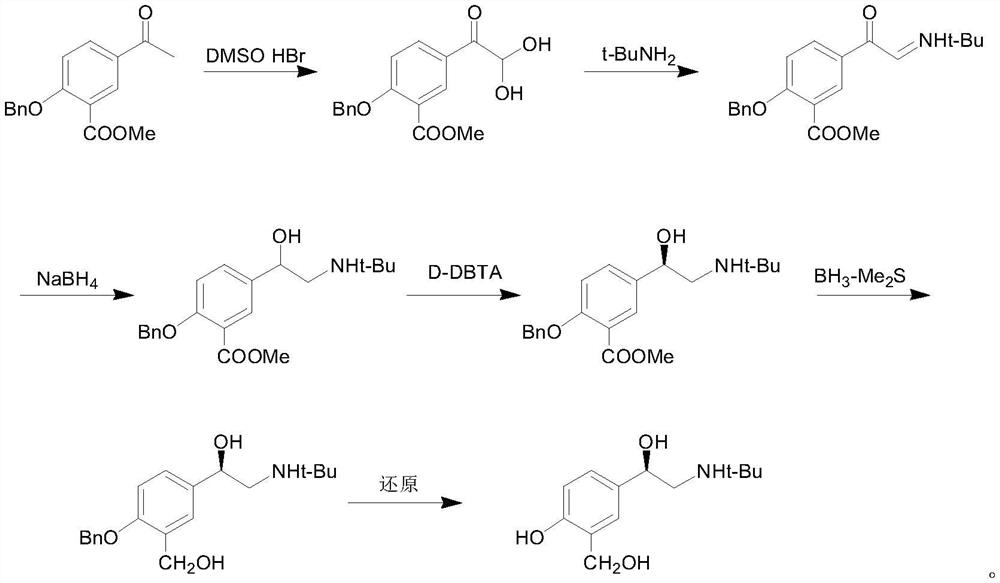
Production technology for synthetizing salbutamol sulphate
InactiveCN104356009AShort reaction stepsMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationChemical synthesisSalicylaldehyde
The invention discloses a production technology for synthetizing salbutamol sulphate, and relates to the field of chemical synthesis. The production technology comprises the following steps: firstly, performing an Friedel-crafts acylation reaction on salicylal to obtain a chemical compound 3, performing a substitution reaction of tert-butylamine, protecting dihydroxy by propylidene under the catalysis of concentrated sulfuric acid through the reduction of potassium borohydride or sodium borohydride, then performing extraction for desalting, and generating a hydrolysis reaction under acid conditions to obtain sulphuric acid salbutamol. The production technology disclosed by the invention is easy to control and operate, raw materials are simple and easy to obtain, the total mol yield is as high as 40%, the product purity is as high as 99.5%, and the product cost is low.
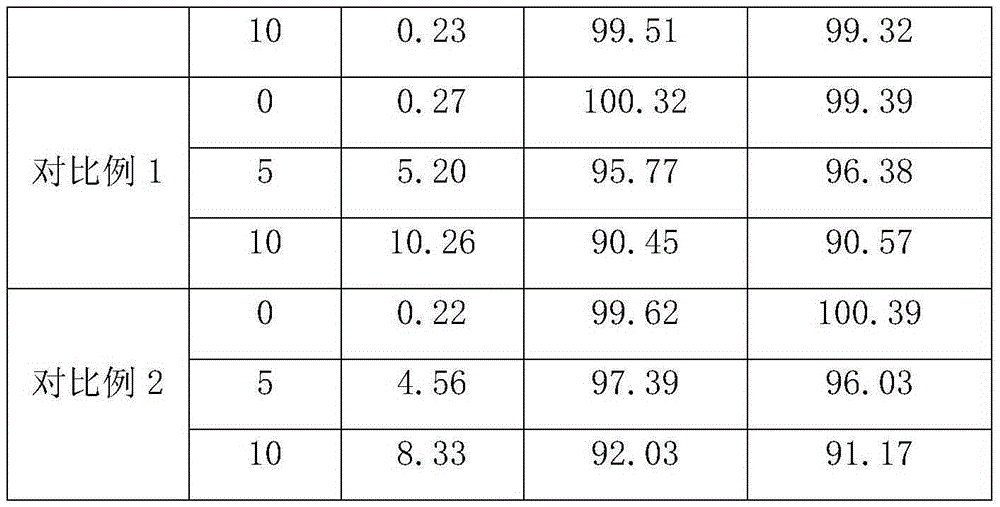
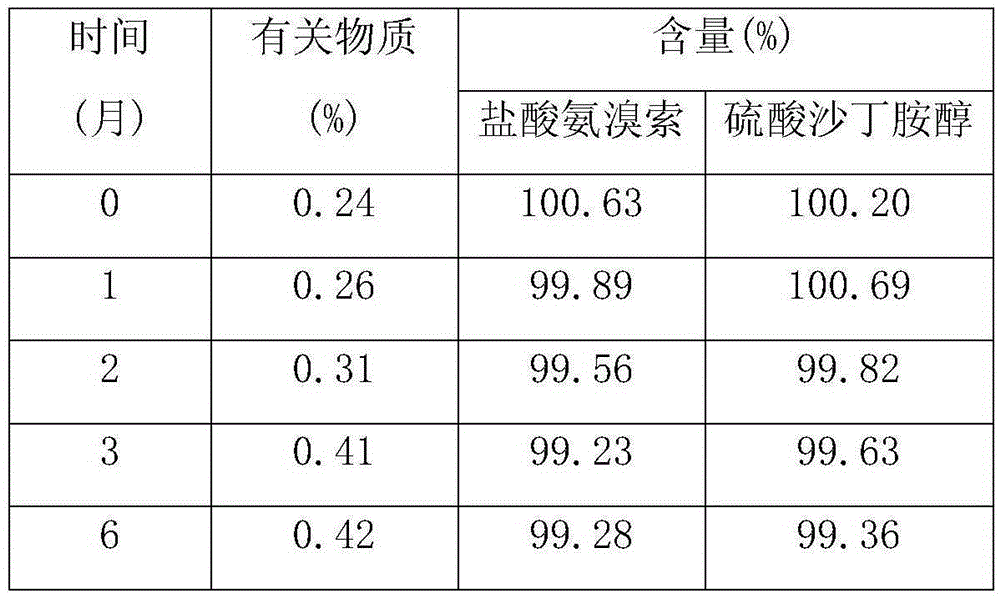
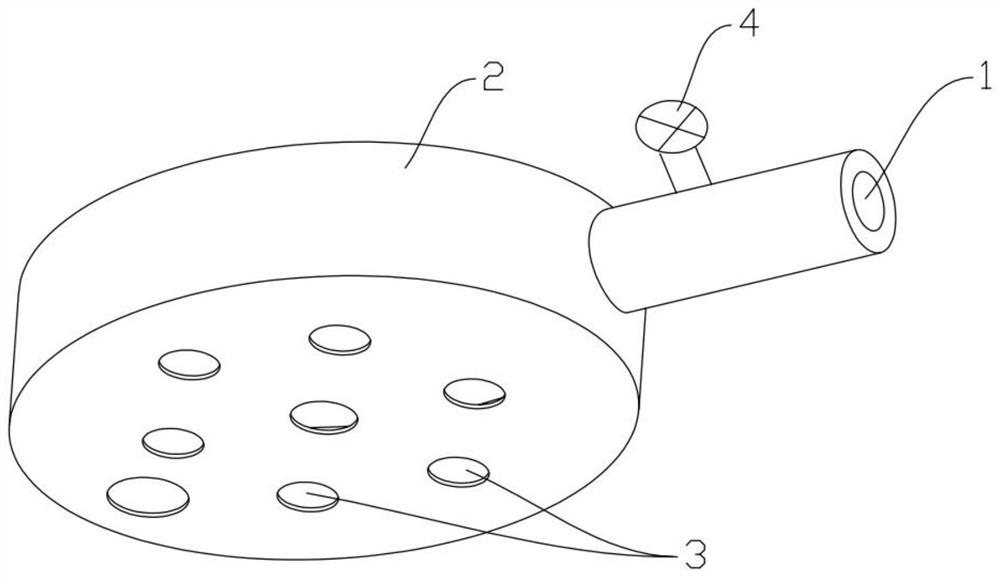
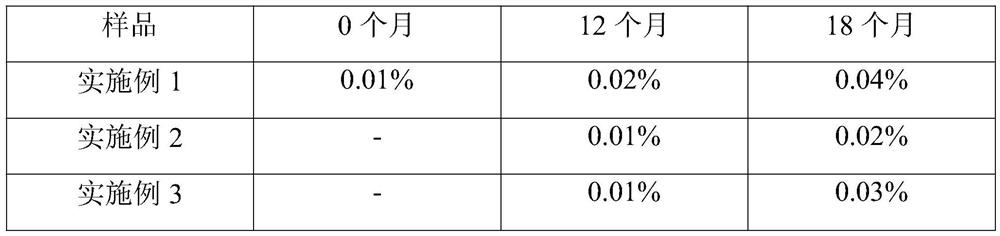
Owner:扬州市三药制药有限公司
Medicaments
This invention relates to aerosol formulations of use for the administration of medicaments by inhalation. More particularly, the invention relates to a pharmaceutical aerosol formulation which comprises particulate salbutamol sulphate having a crystalline form in which the outer layer of the crystals is substantially non-amorphous; and 1,1,1,2-tetrafluoroethane. A method of treating respiratory disorders which comprises administration by inhalation of an effective amount of a pharmaceutical aerosol formulation as defined is also described.
Owner:SMITHKLINE BECKMAN CORP
Pharmaceutical composition for preventing and treating COVID-19 and preparation method of pharmaceutical composition
ActiveCN111265500AGood treatment effectImprove toleranceOrganic active ingredientsDispersion deliveryBULK ACTIVE INGREDIENTSalbutamol sulfate
The invention belongs to the technical field of medicines. The invention relates to a pharmaceutical composition, in particular to a pharmaceutical composition for preventing and treating COVID-19 (diseases caused by novel coronaviruses) and a preparation method thereof. The pharmaceutical composition comprises active ingredients of mycobacterium vaccae for injection and salbutamol sulfate, and the weight ratio of the mycobacterium vaccae for injection to the salbutamol sulfate is 1: (50-200). The medicine prepared from the medicine composition can adopt an aerosol inhalation treatment mode, and through short-term aerosol inhalation, the compliance of a patient can be improved by providing the medicine with a specific purpose for the patient, so that the patient can take the medicine conveniently, and the medical cost is reduced. The pharmaceutical composition for preventing and treating COVID-19 disclosed by the invention has the advantages that the COVID-19 can be effectively prevented and treated; severe or critical symptoms of patients are relieved, nucleic acid negative conversion time is shortened by about 4 days averagely, treatment time is correspondingly shortened, other respiratory virus infections can be prevented and treated nonspecifically, and a new way and scheme are provided for clinical prevention and treatment of COVID-19 and respiratory virus infections.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
Tablet containing ambroxol hydrochloride and salbutamol sulfate
InactiveCN104622854AMethod Prescription is simpleSimple prescriptionOrganic active ingredientsPharmaceutical non-active ingredientsAsthmatic bronchitisDisease
The invention provides a tablet containing ambroxol hydrochloride and salbutamol sulfate, and belongs to the technical field of medicines. The tablet provided by the invention mainly comprises the following auxiliary materials: a filler, a disintegrating agent, a lubricant and an adhesive. The tablet provided by the invention is capable of treating diseases of a respiratory system, such as acute and chronic bronchitis, asthmatic bronchitis and bronchial asthma; sputum is easy to cough up when trachea is expanded; the tablet is simple in prescription, and good in curative effect; and the preparation technology is suitable for industrialized mass production.
Owner:CP PHARMA QINGDAO CO LTD
Salbutamol sulfate sustained-release aerosol of micropowder for inspiration and preparation method thereof
The invention relates to the field of medicinal preparations, in particular to salbutamol sulfate sustained-release aerosol of micropowder for inspiration and a preparation method thereof. The invention is characterized in that the salbutamol sulfate sustained-release aerosol of micropowder for inspiration consists of salbutamol sulfate, polyhydroxyl sugar alcohol, a release sustaining material and amino acid. On the basis of the conventional aerosol of micropowder for inspiration, the sustained-release aerosol of micropowder for inspiration is prepared by adding certain materials. The obtained product has in-bronchus sustained-release performance, has high dissolubility, high bulk density, good angle of repose and high atomization performance and can prevent absorbing moisture.
Owner:CHINA PHARM UNIV
Buccal tablets taking salbutamol sulfate and ambroxol hydrochloride as main active ingredients and preparation method thereof
InactiveCN101810590ASlow onsetReduced bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsTreatment effectOlder people
The invention relates to buccal tablets taking salbutamol sulfate and ambroxol hydrochloride as main active ingredients and a preparation method thereof, and aims to provide a new preparation, namely salbutamol sulfate and ambroxol hydrochloride buccal tablets, for the vast number of patients and medical workers. The preparation has quick response, can improve the bioavailability of medicaments, give full play to the treatment effects of the medicaments and reduce adverse reactions, is convenient for children to swallow and for old people to take with reduced dosage, and is convenient to carry, store and transport. The preparation method is simple and suitable for mass production. In the method, the buccal tablets are prepared by adding some specific types of auxiliary materials in certain proportions into the salbutamol sulfate and ambroxol hydrochloride serving as the active ingredients according to the conventional technique of pharmaceutical engineering.
Owner:北京利乐生制药科技有限公司
Method for synchronously detecting five related substances in compound ipratropium bromide solution for inhalation
ActiveCN111721845AMeet the requirementsAvoid replacementComponent separationAgainst vector-borne diseasesPhosphateSilica gel
The invention relates to a method for synchronously detecting five related substances in a compound ipratropium bromide solution for inhalation, and belongs to the technical field of drug quality determination methods. High performance liquid chromatography is adopted for detection, a chromatographic column with alkyl bonded silica gel is adopted as a filler, a phosphate buffer solution containingsodium heptanesulfonate is used as a mobile phase A, an organic phase is used as a mobile phase B, and a detection wavelength is 205-300nm. The detection method provided by the invention can effectively separate ipratropium bromide and salbutamol sulfate from other impurities, and has characteristics of simple operation, a comprehensive result, accuracy, reliability and strong specificity.
Owner:LUNAN PHARMA GROUP CORPORATION
Salbutamol sulphate inhalation aerosol and preparation method thereof
The invention relates to an inhalation aerosol medicine composition containing selective beta2-receptor stimulant salbutamol sulphate and a hydrofluoroalkane (HFA) propellant and a preparation method thereof. The inhalation aerosol medicine composition and the preparation method have the advantages that the preparation technology is simple and feasible, used raw materials and auxiliary materials have no toxic or side effects, the production cost is low, the prepared medicine has good particle size distribution and relatively high stability, and the quality and the human body bioavailability of the medicine are favorably improved.
Owner:江苏山信药业有限公司 +1
Compositions comprising salbutamol sulphate
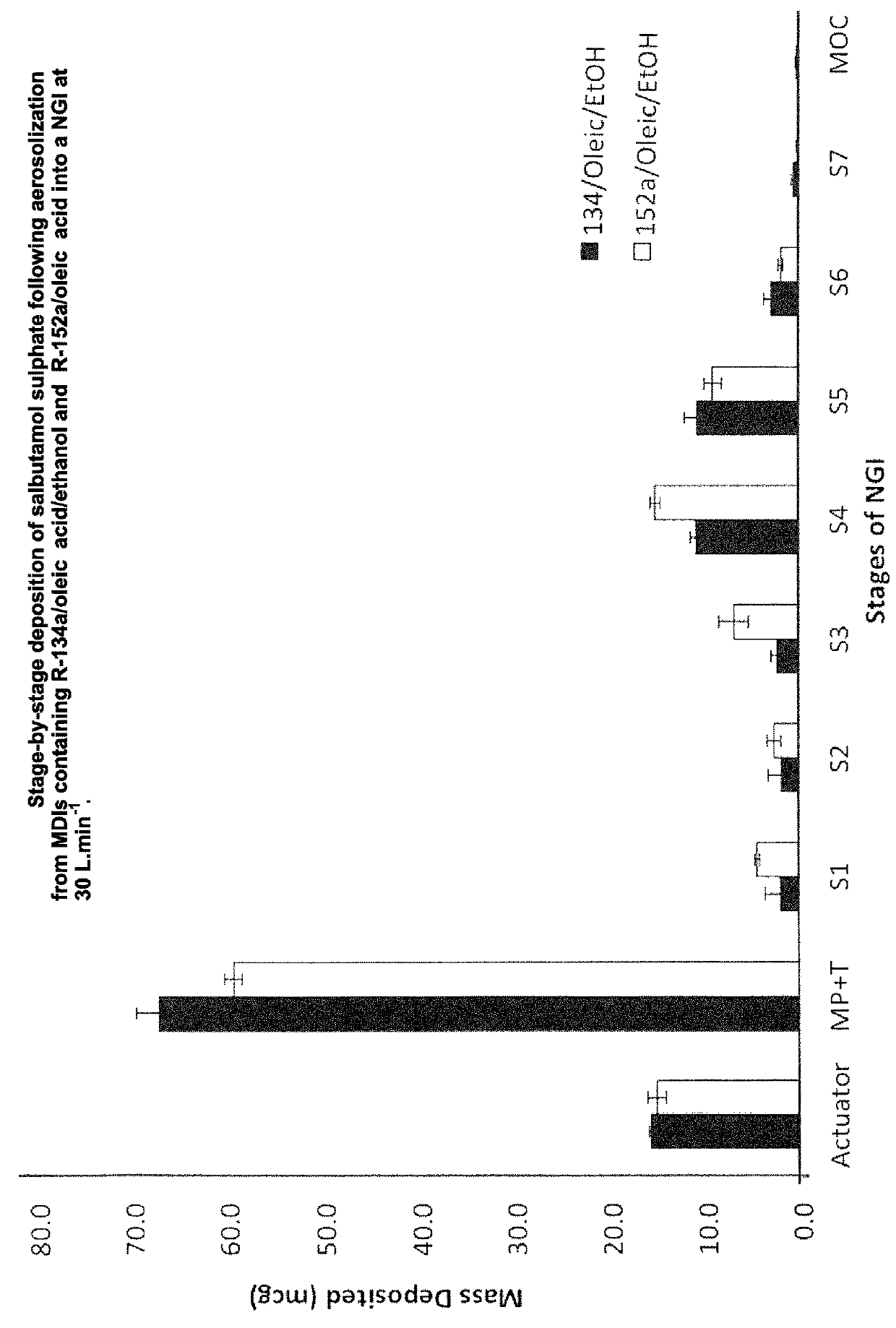
A pharmaceutical composition is described that is suitable for delivery from a pressurised container. The composition is free of polar excipients and comprises: (a) a propellant component that consists essentially of 1,1-difluoroethane (R-152a); (b) a surfactant component that comprises oleic acid; and (c) a drug component that consists of salbutamol sulphate. The pharmaceutical composition can be delivered using a metered dose inhaler (MDI).
Owner:MEXICHEM AMANCO HLDG DE C V
Salbutamol sulfate aerosol inhalation solution, and preparation process and application thereof
PendingCN112402400APromote atomizationImprove thermal stabilityOrganic active ingredientsDispersion deliveryMedicineAerosolize
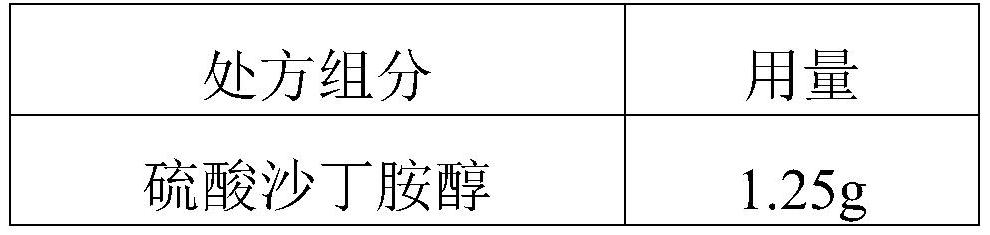
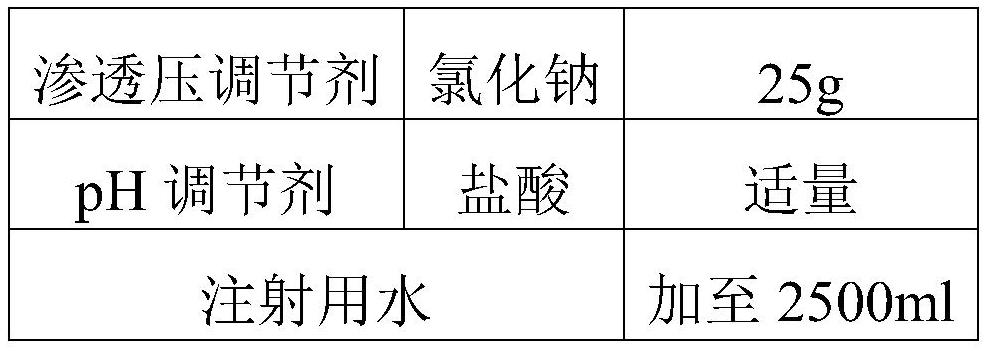
The invention discloses a salbutamol sulfate aerosol inhalation solution, and a preparation process and application thereof. The salbutamol sulfate solution is prepared from the following components:salbutamol sulfate, an osmotic pressure regulator, a pH regulator and water for injection, wherein the pH value of the salbutamol sulfate solution is 3.0 to 5.0. The salbutamol sulfate solution has good atomization performance and thermal stability.
Owner:SHANGHAI SINE PHARMA LAB
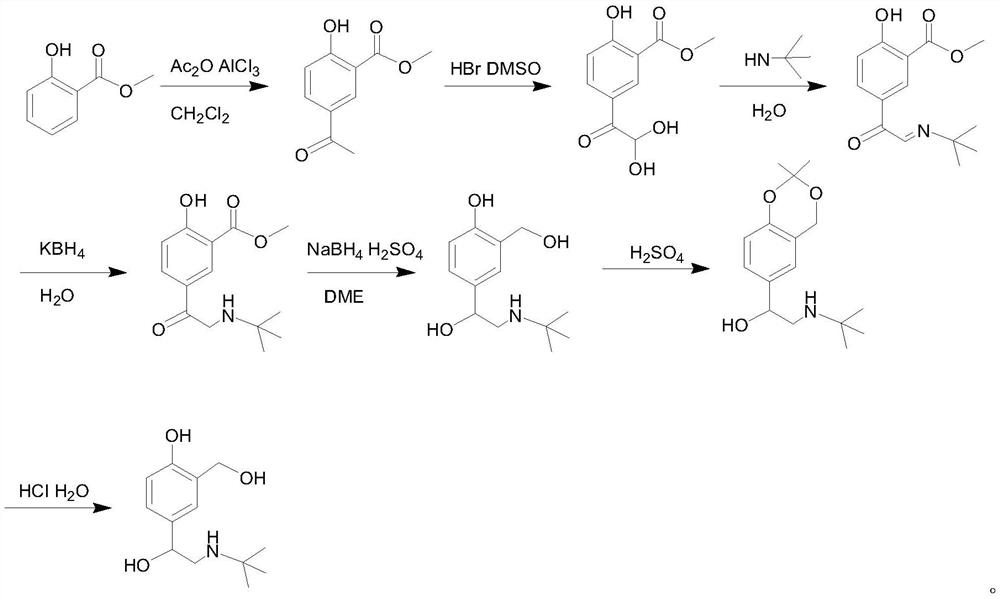
Salbutamol sulfate impurity and preparation method thereof
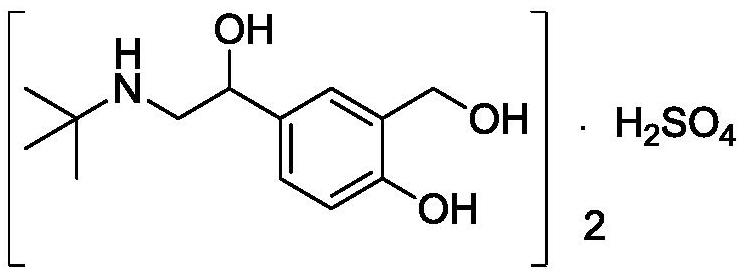
PendingCN110981740ASimple and fast operationThe reaction conditions are mild and controllableOrganic compound preparationAmino-hyroxy compound preparationPhenolSalbutamol sulfate
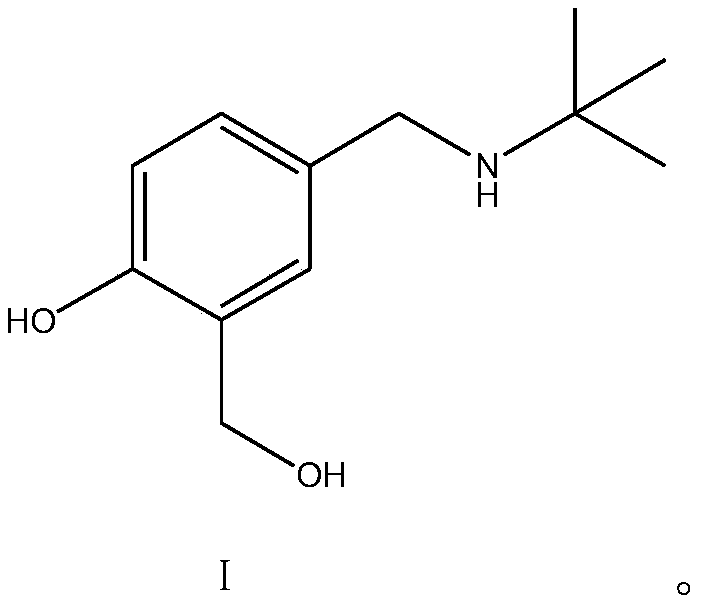
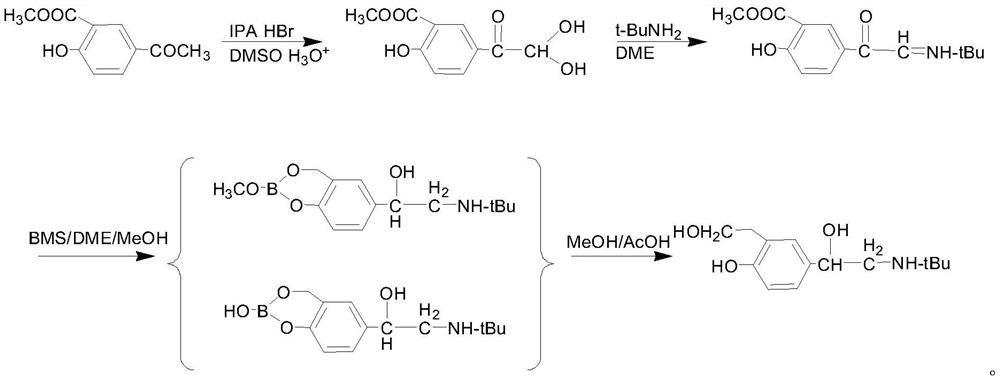
The invention discloses a salbutamol sulfate impurity and a preparation method thereof, the chemical name of the salbutamol sulfate impurity is (4-[(tert-butylamino) methyl]-2-(hydroxymethyl) phenol),and the salbutamol sulfate impurity is prepared by carrying out Schiff base reaction on a compound shown as a formula II and tert-butylamine under the action of a dehydrating agent to obtain a compound shown as a formula III, and carrying out reduction reaction on the compound shown in a formula III under the action of a reduction catalyst to prepare the compound shown in a formula I, namely (4-[(tert-butylamino) methyl]-2-(hydroxymethyl) phenol). The preparation method of the salbutamol sulfate impurity disclosed by the invention is simple and convenient to operate, mild and controllable inreaction condition, good in reaction repeatability, high in product yield and high in purity; an impurity reference substance meeting the requirements is provided for quality control of salbutamol sulfate, and the impurity reference substance can be used for quality research of salbutamol sulfate bulk drugs and drugs thereof, so that the salbutamol sulfate bulk drugs and the drugs thereof meet related substance standards.
Owner:ANHUI HEALSTAR PHARM CO LTD
Medicaments
This invention relates to aerosol formulations of use for the administration of medicaments by inhalation. More particularly, the invention relates to a pharmaceutical aerosol formulation which comprises particulate salbutamol sulphate having a crystalline form in which the outer layer of the crystals is substantially non-amorphous; and 1,1,1,2-tetrafluoroethane. A method of treating respiratory disorders which comprises administration by inhalation of an effective amount of a pharmaceutical aerosol formulation as defined is also described.
Owner:SMITHKLINE BECKMAN CORP
Preparation method of salbutamol sulfate
PendingCN113121369AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisPtru catalyst
The invention provides a preparation method of salbutamol sulfate, and relates to the technical field of chemical synthesis. The preparation method of salbutamol sulfate comprises the following steps: (a) carrying out reaction on salicylic acid and a tert-butyl amino acetyl halogenating reagent under the action of a chlorinating agent to generate an intermediate 1; (b) reacting the intermediate 1 under the action of Lewis acid to generate an intermediate 2; (c) reacting the intermediate 2 under the action of an acid reagent to generate an intermediate 3; (d) reacting the intermediate 3 with a reducing agent under the action of a catalyst to generate salbutamol; and (e) reacting salbutamol with sulfuric acid to generate salbutamol sulfate. The raw materials are simple and easy to obtain, the reaction condition is mild, the reaction operation is simple, the reaction process is easier to control, and the safety coefficient is high; used raw materials, solvents and the like are environment-friendly; and the yield and purity of salbutamol sulfate are high, and a process route capable of industrially producing a product with higher quality is provided.
Owner:TIANJIN PHARMA GROUP CORP
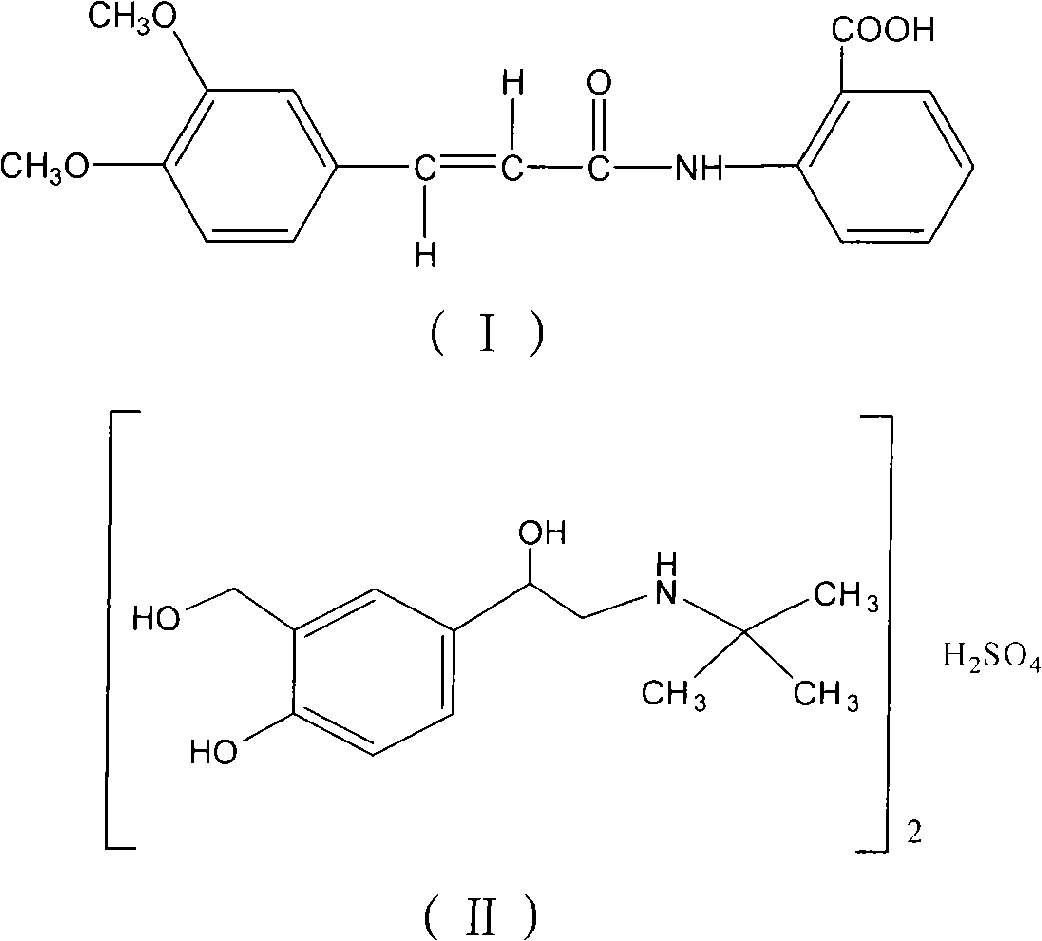
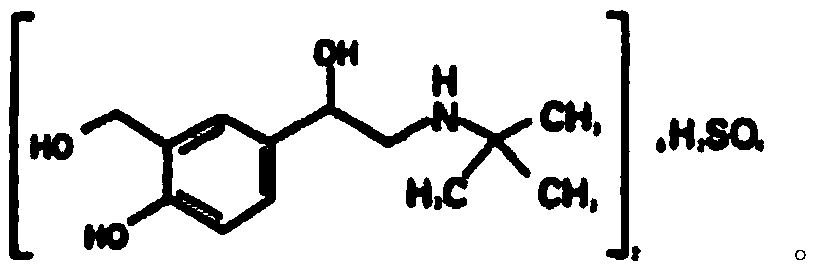
Oral compound pharmaceutic preparation containing tranilast and salbutamol
InactiveCN101683330AOrganic active ingredientsPharmaceutical delivery mechanismOral treatmentBULK ACTIVE INGREDIENT
The invention relates to an oral compound pharmaceutic preparation for treating asthma, which contains tranilast shown as the formula (I) and salbutamol sulfate shown as the formula (II) or other pharmaceutically allowed active ingredients. The invention also relates to the application of compounds shown as formulas (I) and (II) or pharmaceutically allowed salts thereof in the preparation of an oral therapeutical agent for treating asthma.
Owner:沈阳三川医药科技有限公司
Salbutamol sulfate oral cavity disintegration tablets and preparation method thereof
InactiveCN111228227AReduce usageLow costOrganic active ingredientsPill deliveryPharmaceutical AidsTableting
The invention provides salbutamol sulfate oral cavity disintegration tablets and a preparation method thereof. The preparation method comprises the following steps of weighing a raw material namely salbutamol sulfate and auxiliary materials, performing screening, premixing the raw material with the auxiliary materials, and performing tabletting, wherein a powder direct tabletting technology is adopted for tabletting. The preparation method disclosed by the invention has the following advantages that the direct tabletting technology is adopted, fewer working procedures are adopted, equipment issimple, and commercial production is easy; after the particle diameter of the auxiliary materials is controlled, the fluidity and the compressibility of granules are greatly improved; after the technology is improved, the impurity level is lower than that of an original product; and the safety of a patient is improved, and potential safety hazards are avoided.
Owner:CHONGQING CONQUER PHARML
Salbutamol sulfate solution for inhalation and preparation method thereof
ActiveCN110898042AImprove toleranceIncreased maximum relaxation ratePharmaceutical delivery mechanismPharmaceutical non-active ingredientsInhalationIpratropium bromide
The invention provides a salbutamol sulfate solution for inhalation and a preparation method thereof. The prescription of the salbutamol sulfate solution comprises salbutamol sulfate, ipratropium bromide, an isoosmotic adjusting agent, a pH adjusting agent, sodium hyaluronate, carboxymethyl chitosan oligosaccharide and water for injection. The preparation process comprises the following steps: weighing, preparing a solution, adjusting the pH value, filtering, encapsulating, carrying out quality inspection and the like. According to the prescription and the preparation method, the stability ofthe preparation can be improved, meanwhile, the preparation has a good permeation effect, the salbutamol sulfate tolerance can be improved, and the preparation can better play a role.
Owner:深圳大佛药业股份有限公司
Salbutamol sulfate solution for inhalation and preparation method thereof
ActiveCN113476428AReduce the introductionSimple prescriptionOrganic active ingredientsPharmaceutical delivery mechanismDrug contentTherapeutic effect
The invention discloses a salbutamol sulfate solution for inhalation and a preparation method thereof. A formula of the salbutamol sulfate solution comprises salbutamol sulfate, lactose, an isotonicity regulator, a pH adjusting agent, an antioxidant and the balance of water for injection. In a preparation process, carbon dioxide is introduced as the antioxidant, so that the dissolved oxygen content and the residual oxygen content in the solution are reduced. The added lactose not only is beneficial to the flowability of the medicine component salbutamol sulfate, but also can be used for preventing the salbutamol sulfate from being agglomerated together, so that the salbutamol sulfate can be uniformly dispersed in a solution system. According to the salbutamol sulfate solution for inhalation, the impurity content is low, adverse effects of related substances on human health and drug effects are reduced, the stability is higher, the drug content distribution is uniform, and the treatment effect is facilitated.
Owner:朗天药业(湖北)有限公司 +1
Preparation method of ambroxol and salbutamol enteric coatel tablet
InactiveCN105434390AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMannitolBioavailability
The invention relates to an ambroxol and salbutamol enteric coatel tablet, and belongs to the field of pharmaceutic preparations. Due to poloxamer 188, meglumine and mannitol which are appropriately added to the enteric coatel tablet, the stability and bioavailability of ambroxol hydrochloride and salbutamol sulfate in the preparation can be remarkably improved, quality is stable, the effect is remarkable, and respiratory system diseases such as acute and chronic bronchitis and asthma can be effectively treated.
Owner:CP PHARMA QINGDAO CO LTD
Compound tranilast orally disintegrating tablet formulation and preparation method thereof
InactiveCN101375843ASolve the respective defects in pharmacological actionEasy to takeOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletBioavailability
The invention aims to provide a compound oral disintegrating tablet preparation which is composed of tranilast and salbutamol sulfate, and a preparation method thereof. The adopted specific technical proposal for achieving the purpose of the invention is as follows: the compound tranilast oral disintegrating tablet preparation is characterized in that the formula of the preparation is composed of the following components by parts by weight (by 1000 tablets): 80.0g of tranilast, 2.4g of salbutamol sulfate, 5-30g of disintegrant, 10-80g of filling agent, 5-25g of effervescing agent, 0.5-20g of flavoring agent and 0.3-3g of lubricant. The compound oral disintegrating tablet preparation which is prepared by the method can solve the shortcomings of the two drugs on the pharmacological effects during the treatment process of bronchial asthma and allow the compound oral disintegrating tablet preparation to relieve the asthma instantly and maintain and consolidate the effect; furthermore, the compound oral disintegrating tablet preparation has convenient administration, rapid onset of action, high bioavailability and good taste. The preparation method of the preparation has simple steps and low cost.
Owner:SHANGHAI NORMAL UNIVERSITY
Salbutamol sulfate inhalation preparation and preparation method thereof
ActiveCN111658661AImprove stabilityAccurate quality controlAntibacterial agentsOrganic active ingredientsUse medicationInhalation preparations
The invention relates to the technical field of a medicine preparation, in particular to a salbutamol sulfate inhalation preparation and a preparation method thereof. Per 1000 ml of the salbutamol sulfate inhalation preparation is prepared from the following ingredients: 0.5 to 2.0 g of salbutamol sulfate, 0.1 to 0.4 g of erythromycin, 0.2 to 0.6 g of L-fucose, 1 to 3 g of a taste-masking agent, 4to 8 g of an osmotic pressure regulator, 1 to 5 g of a pH regulator and the balance of injection water. The salbutamol sulfate inhalation preparation has the advantages that the stability is good under the acid condition; the quality control is accurate; the effect is stable; the mouthfeel is good; and the drug compliance is improved.
Owner:深圳大佛药业股份有限公司
Ambroxol salbutamol controlled release tablet
ActiveCN105362247AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseS albuterol
The invention relates to an ambroxol salbutamol controlled release tablet and belongs to the field of pharmaceutical preparations. The controlled release tablet is suitably added with poloxamer 188, meglumine and mannitol, significant improvement can be given to the stability and bioavailability of ambroxol hydrochloride and salbutamol sulfate in a preparation, the quality is stable, the effect is significant, and effective treatment may be given to respiratory diseases such as acute and chronic bronchitis and asthma.
Owner:CP PHARMA QINGDAO CO LTD
Preparation method of dropping pills containing ambroxol hydrochloride and salbutamol sulfate
ActiveCN105326805AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPill deliveryDiseaseMANNITOL/SORBITOL
The invention relates to dropping pills containing ambroxol hydrochloride and salbutamol sulfate and belongs to the field of medicinal preparations. Poloxamer188, meglumine and mannitol are properly added into the dropping pills so that the stability and bioavailability of ambroxol hydrochloride and salbutamol sulfate in the preparation can be remarkably improved, and the dropping pills have stable quality and a remarkable effect and can effectively treat acute or chronic bronchitis, asthma and other respiratory diseases.
Owner:CP PHARMA QINGDAO CO LTD
A kind of albuterol sulfate solution for inhalation and preparation method thereof
ActiveCN113476428BReduce the introductionSimple prescriptionOrganic active ingredientsPharmaceutical delivery mechanismDrug contentAlcohol
Owner:朗天药业(湖北)有限公司 +1
Oral liquid formulation comprising salbutamol and guaifenesin
ActiveCN102573796BReduce the incidence of allergic reactionsSolution deliveryPharmaceutical non-active ingredientsSalbutamol SulphateAqueous dispersion
Owner:苏鲁・苏布拉马尼・瓦南加穆迪
Preparation method of solution containing ambroxol hydrochloride and salbutamol sulfate
ActiveCN105326790AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismMannitolObstructive chronic bronchitis
The invention relates to a solution containing ambroxol hydrochloride and salbutamol sulfate and belongs to the field of medicinal preparations. Poloxamer188, meglumine and mannitol are properly added into the solution so that the stability and bioavailability of ambroxol hydrochloride and salbutamol sulfate in the preparation can be remarkably improved, and the solution has stable quality and a remarkable effect and can effectively treat acute or chronic bronchitis, asthma and other respiratory diseases.
Owner:CP PHARMA QINGDAO CO LTD
Preparation method of salbutamol sulfate impurities
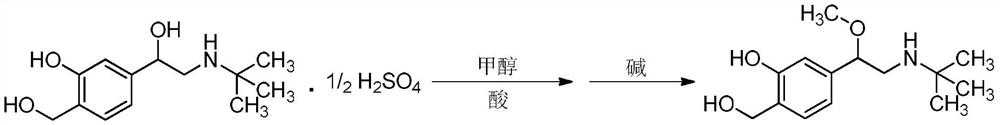
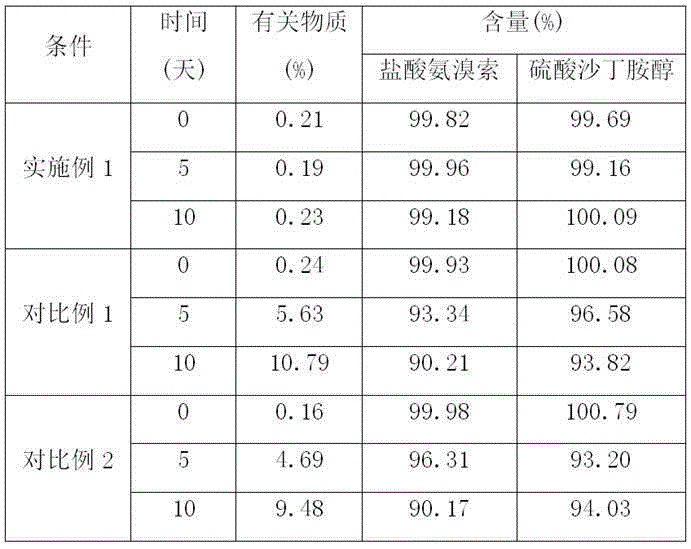
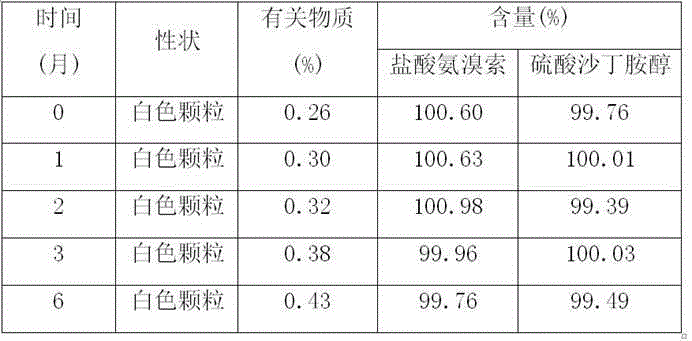
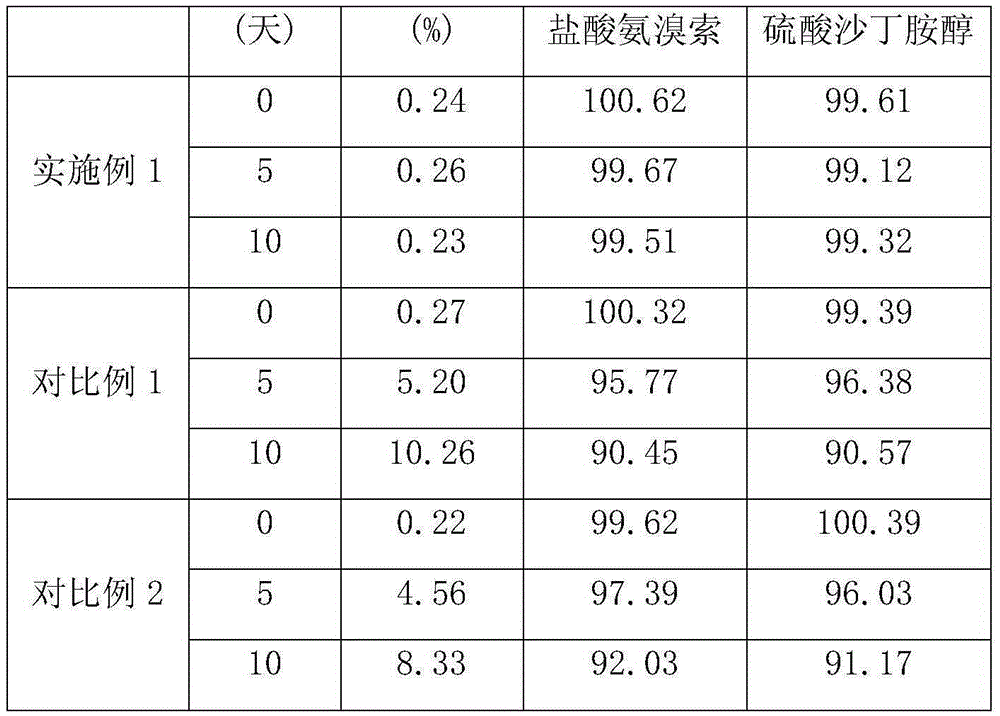
The invention relates to the technical field of chemical synthesis, in particular to a preparation method of salbutamol sulfate impurities. The method comprises the following steps: (1) dissolving salbutamol sulfate in methanol, carrying out etherification reaction under acidic conditions, performing dissociating with alkali, and carrying out column purification to obtain a methyl ether intermediate; and (2) dissolving the methyl ether intermediate in an organic solvent, adding concentrated sulfuric acid to react with a cyclization reagent, carrying out dissociating by using alkali, and performing purifying by using a column to obtain a product. The technical scheme adopted by the invention has the beneficial effects that the operation is convenient, the reaction conditions are mild and controllable, the reaction stability is high, and the reaction product is high in yield and purity. In addition, the compound shown in the formula I can provide an impurity reference substance meeting requirements for quality control of salbutamol sulfate.
Owner:CHANGZHOU YABANG PHARMA
Ambroxol and salbutamol capsule
InactiveCN105412056AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseObstructive chronic bronchitis
The invention relates to an ambroxol and salbutamol capsule, and belongs to the field of a pharmaceutical preparation. The capsule, which is added with proper amounts of poloxamer 188, meglumine and mannitol, can significantly improve the stability and the bioavailability of ambroxol hydrochloride and salbutamol sulfate in a preparation; and the capsule is stable in quality and significant in effect, and the capsule is capable of effectively treating such diseases of respiratory system as acute and chronic bronchitis, asthma and the like.
Owner:CP PHARMA QINGDAO CO LTD
Dropping pills containing ambroxol hydrochloride and salbutamol sulfate
ActiveCN105326804AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMeglumineDisease
The invention relates to dropping pills containing ambroxol hydrochloride and salbutamol sulfate and belongs to the field of medicinal preparations. Poloxamer188, meglumine and mannitol are properly added into the dropping pills so that the stability and bioavailability of ambroxol hydrochloride and salbutamol sulfate in the preparation can be remarkably improved, and the dropping pills have stable quality and a remarkable effect and can effectively treat acute or chronic bronchitis, asthma and other respiratory diseases.
Owner:CP PHARMA QINGDAO CO LTD
Ambroxol salbutamol enteric particles
InactiveCN105362234AHigh content of active ingredientsImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseMedicine
The invention relates to ambroxol salbutamol enteric particles, and belongs to the field of pharmaceutic preparations. A proper amount of poloxamer 188, meglumine and mannitol are added in the enteric particles, stability and bioavailability of ambroxol hydrochloride and salbutamol sulfate in preparations can be improved remarkably, the quality is stable, the effect is remarkable, and respiratory system diseases such as acute and chronic bronchitis and asthma can be treated effectively.
Owner:CP PHARMA QINGDAO CO LTD
Method for refining salbutamol sulfate
InactiveCN105001100AHigh purityStable traitsOrganic compound preparationAmino-hyroxy compound preparationCentrifugationRoom temperature
The invention relates to a method for refining salbutamol sulfate and belongs to the technical field of fine chemical separation and purification. The method for refining the salbutamol sulfate aims to achieve the technical purposes that the technique is simple and the purity of refined products is high. According to the technical scheme, the method for refining the salbutamol sulfate comprises the steps that crude salbutamol sulfate is dissolved in purified water, filtering is conducted, and thus salbutamol sulfate filter liquor is obtained for later use, wherein the weight ratio of the salbutamol sulfate and water is 1:3.6; acetone with the weight being three times to four times that of the crude salbutamol sulfate is added into the filter liquor, and crystallization is conducted for four hours at the normal temperature; crystallized liquor is poured into a centrifugal machine, the liquor is added multiple times in the way that a small amount of liquor is added each time, the centrifugal machine is started till no liquor flows out from a discharge outlet, and a filter cake is washed by means of acetone till the filter liquor is clear; centrifugation continues to be conducted for half an hour, the centrifugal machine is stopped, and discharging is conducted; a centrifuged wet product is contained in a clean stainless steel tray and evenly spread flat, and the acetone is naturally dried for more than three hours; the tray is placed in a drying box to be dried for more than four hours by controlling the temperature to be 70-80 DEG C, and then sieving is conducted by means of a 40-mesh sieve.
Owner:SHANXI YUNPENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com