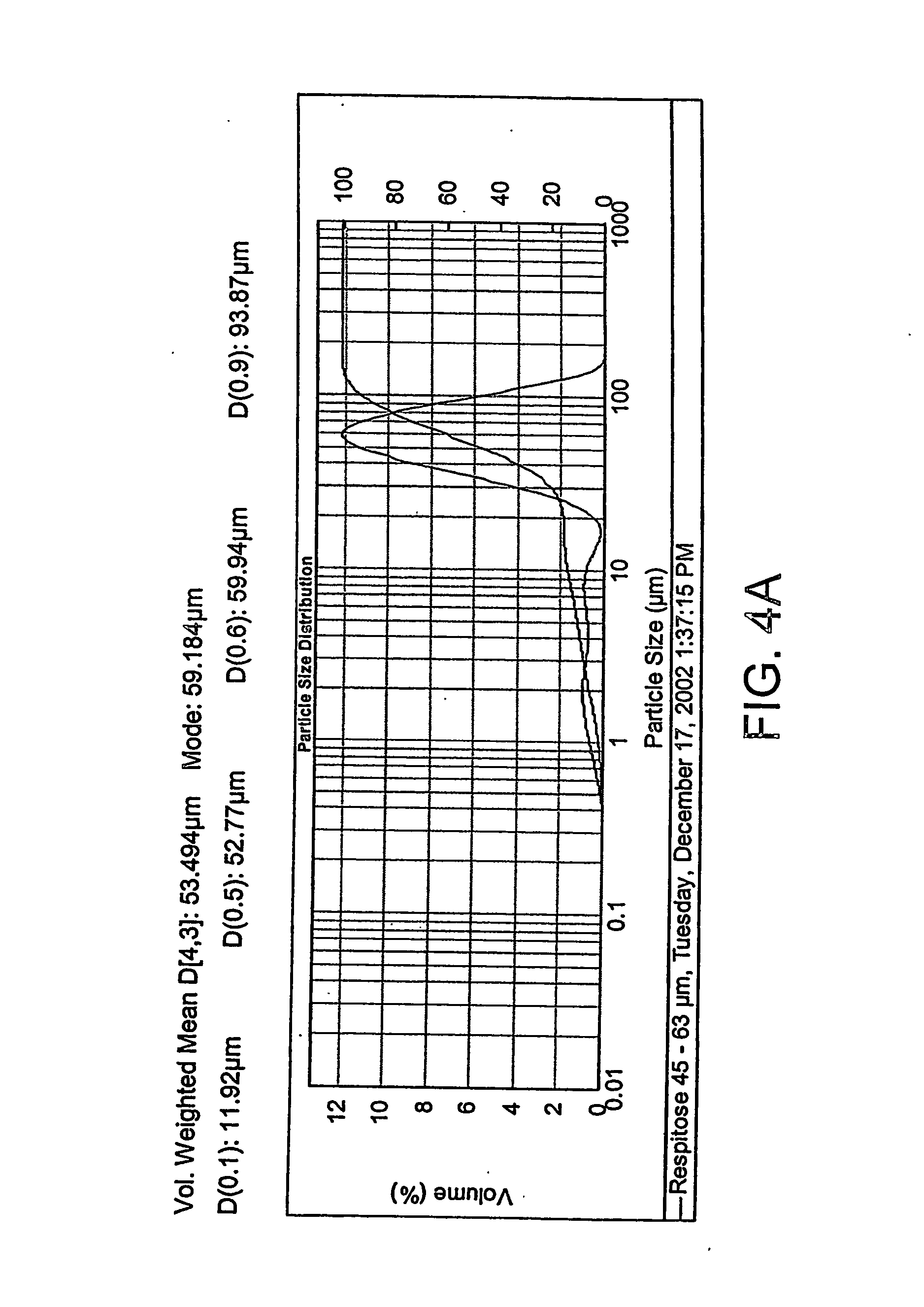
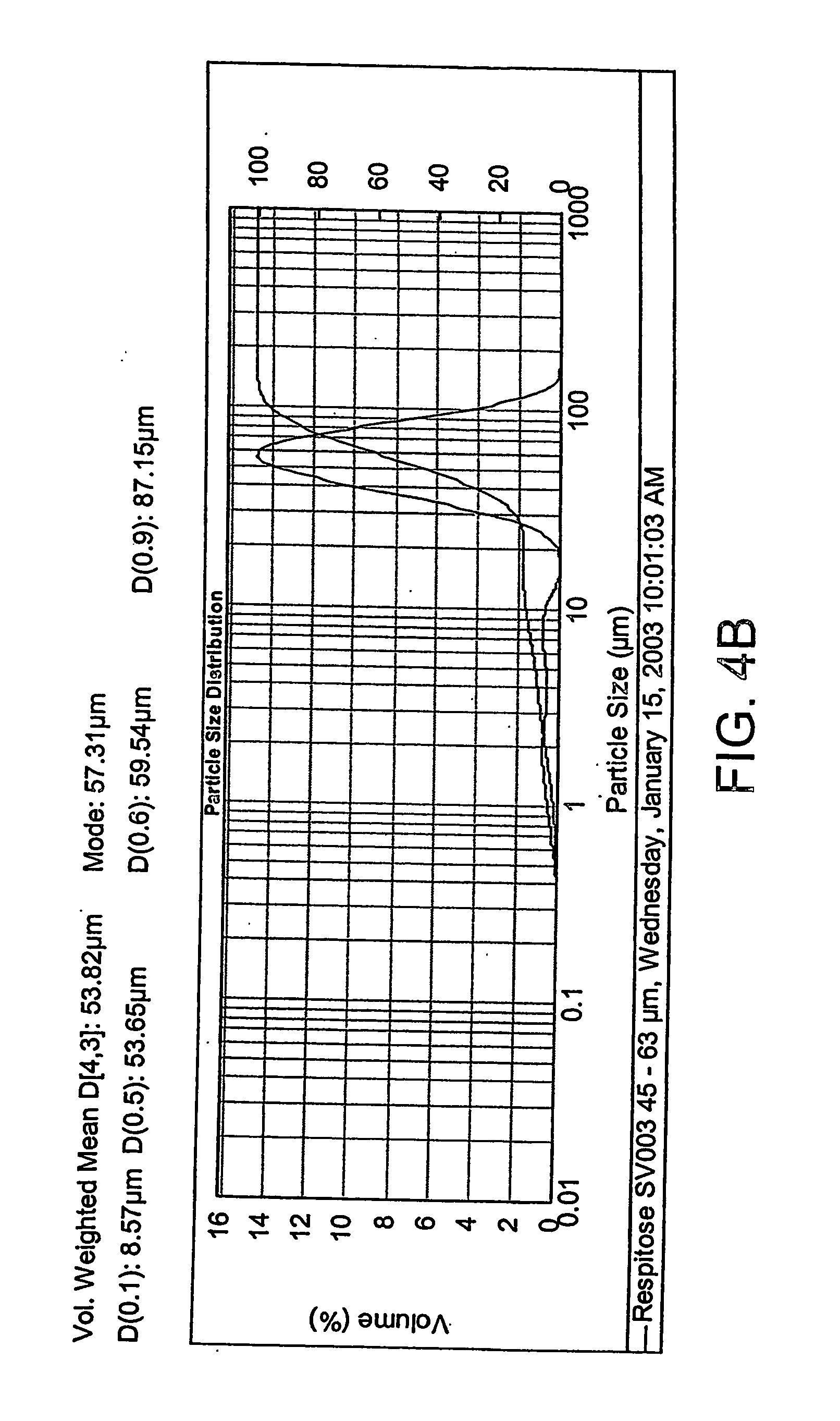
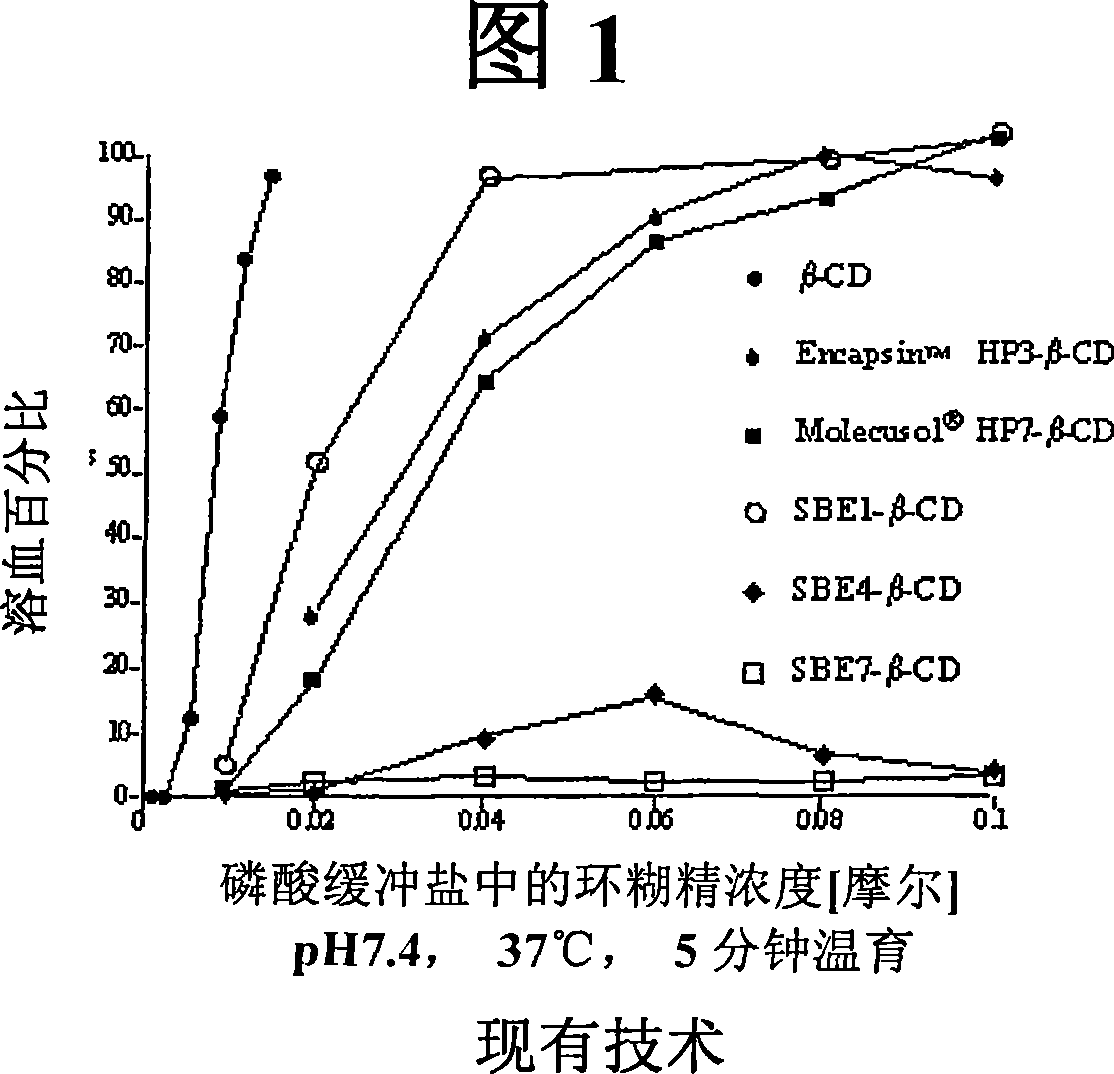
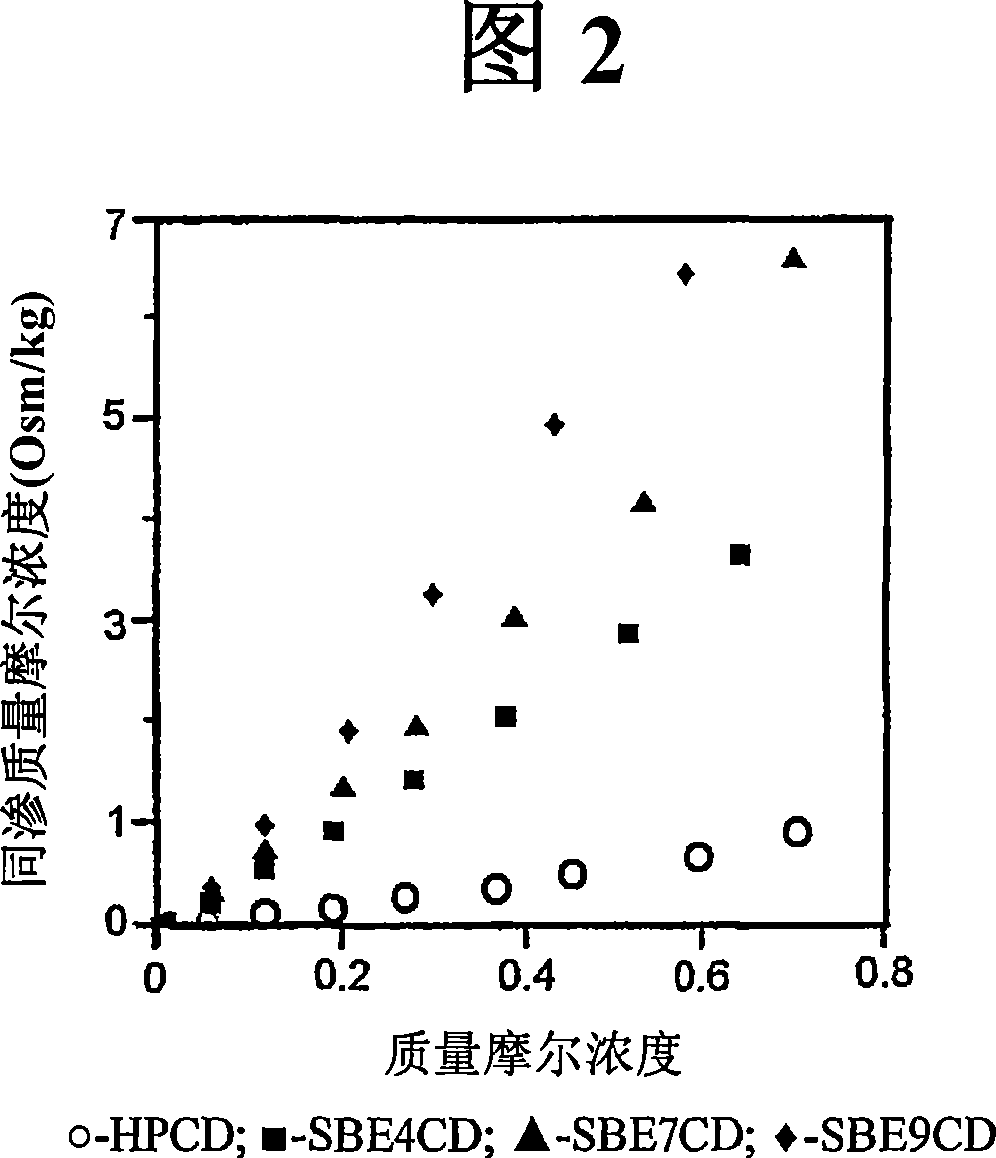
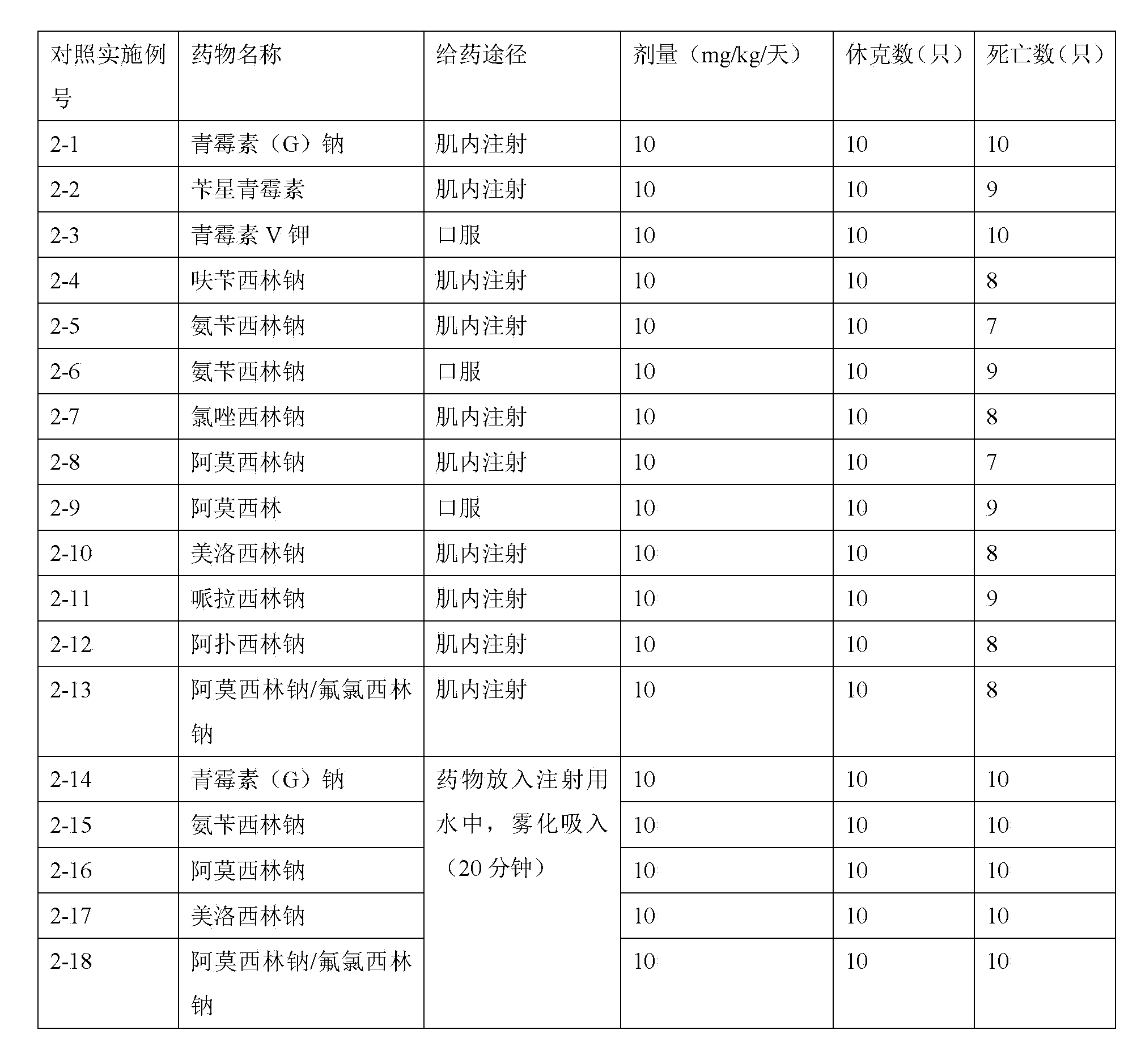
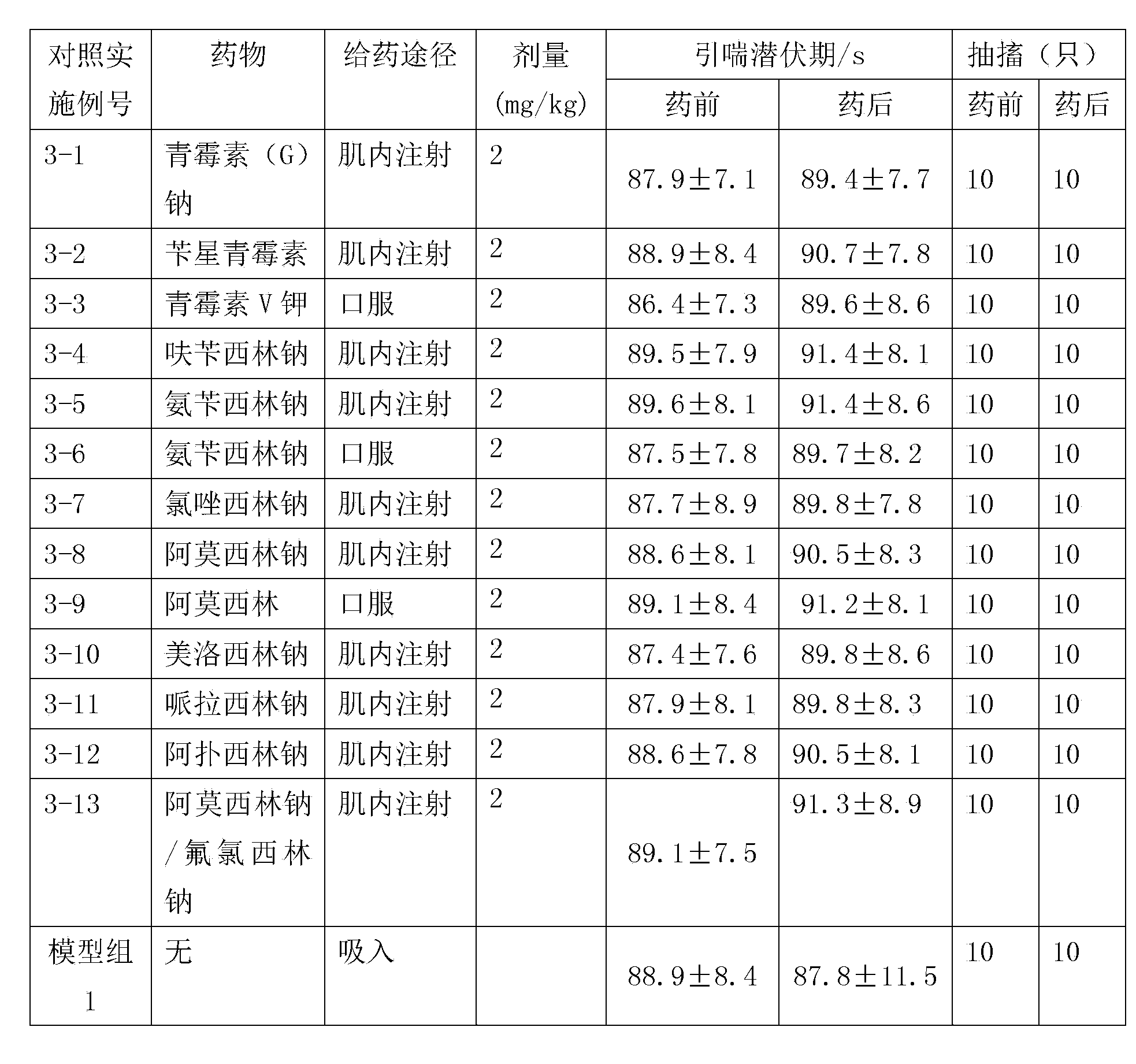
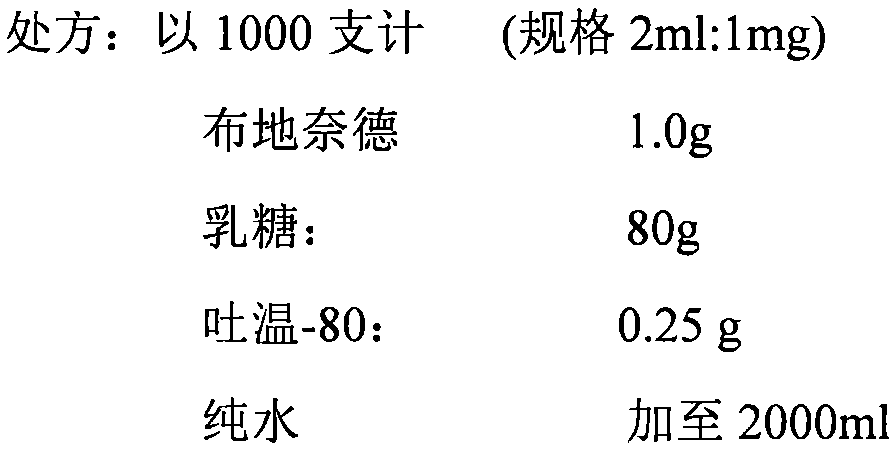
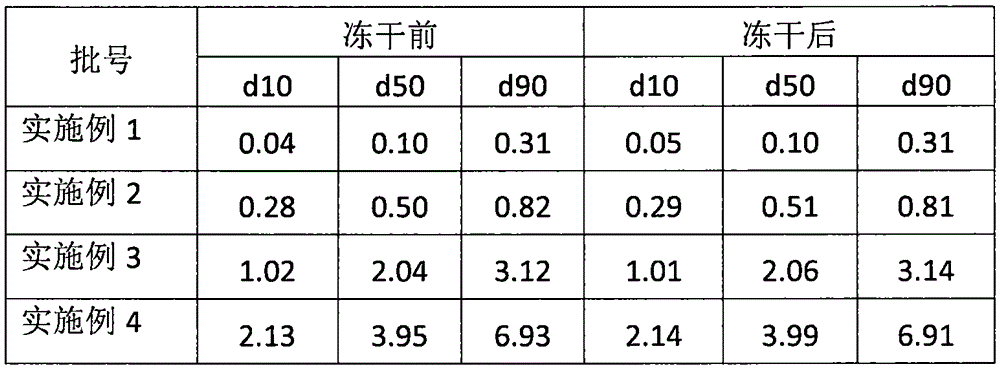
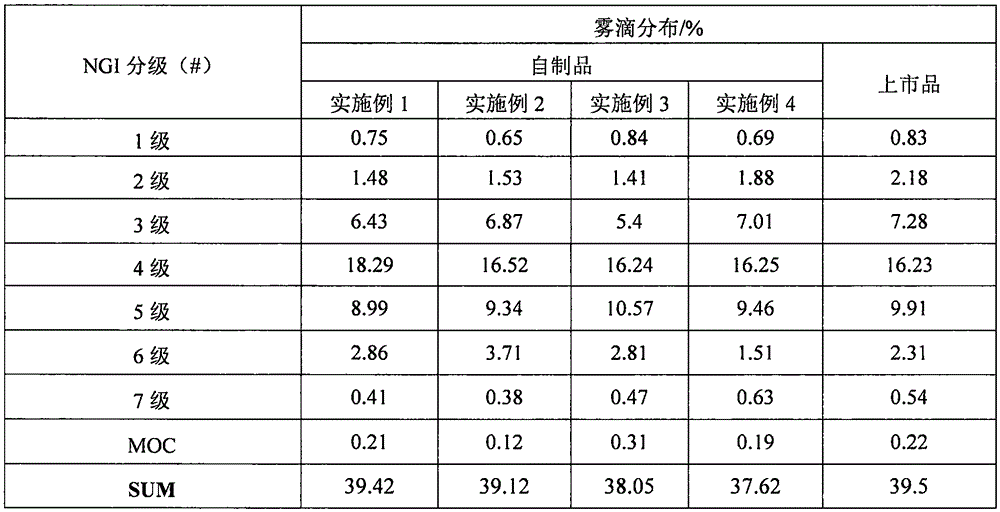
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101 results about "Inhalation preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
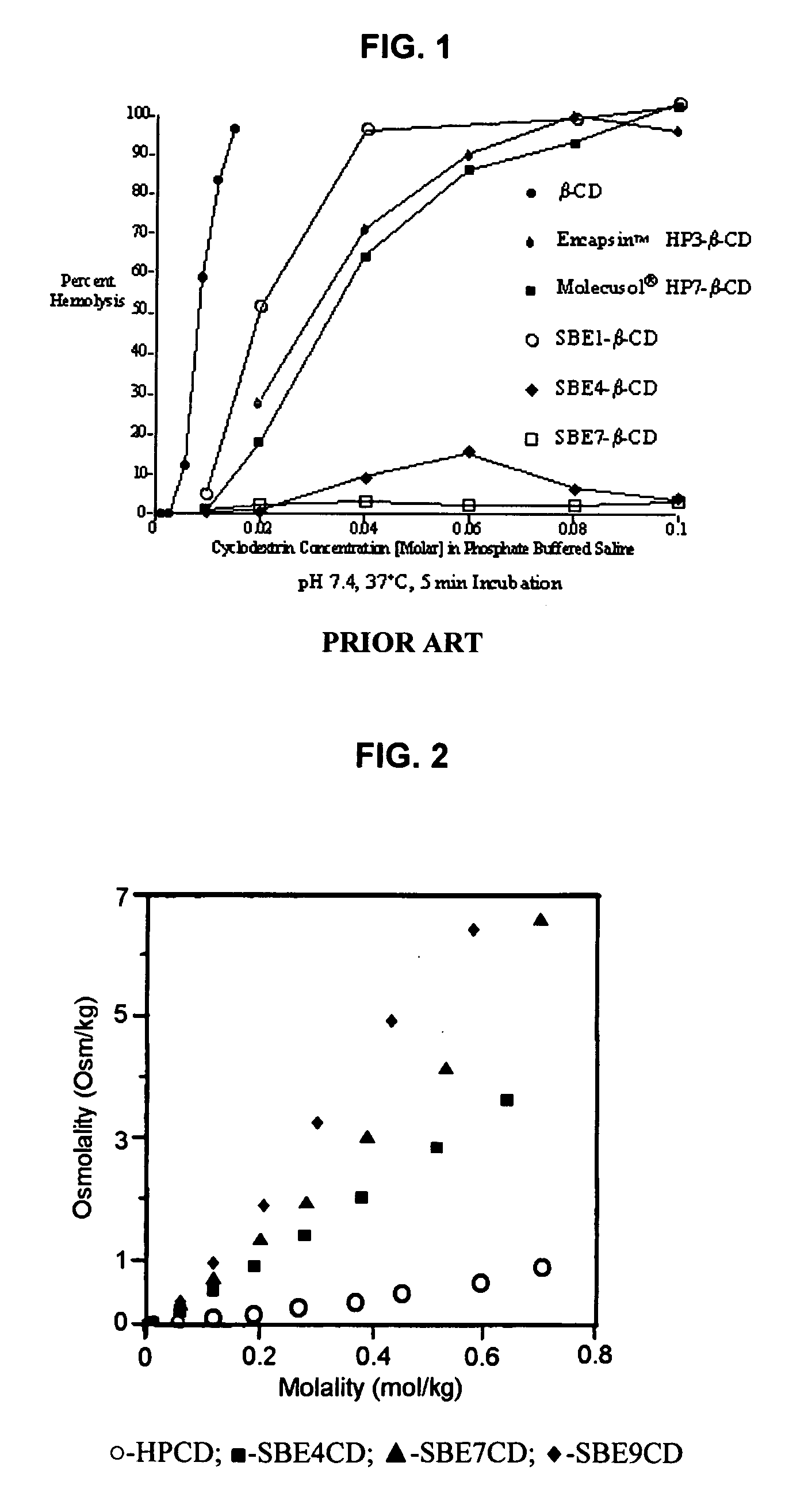
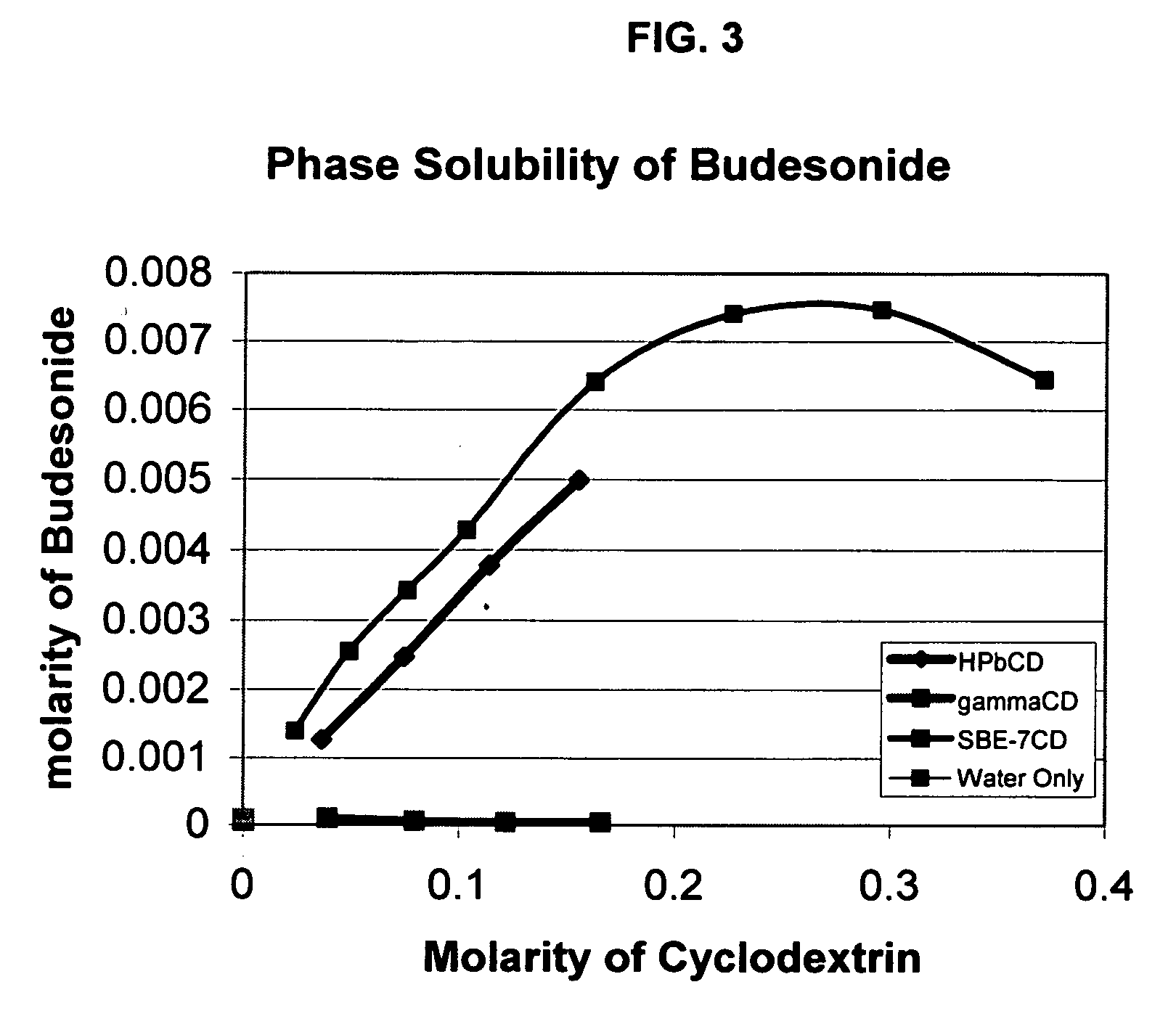
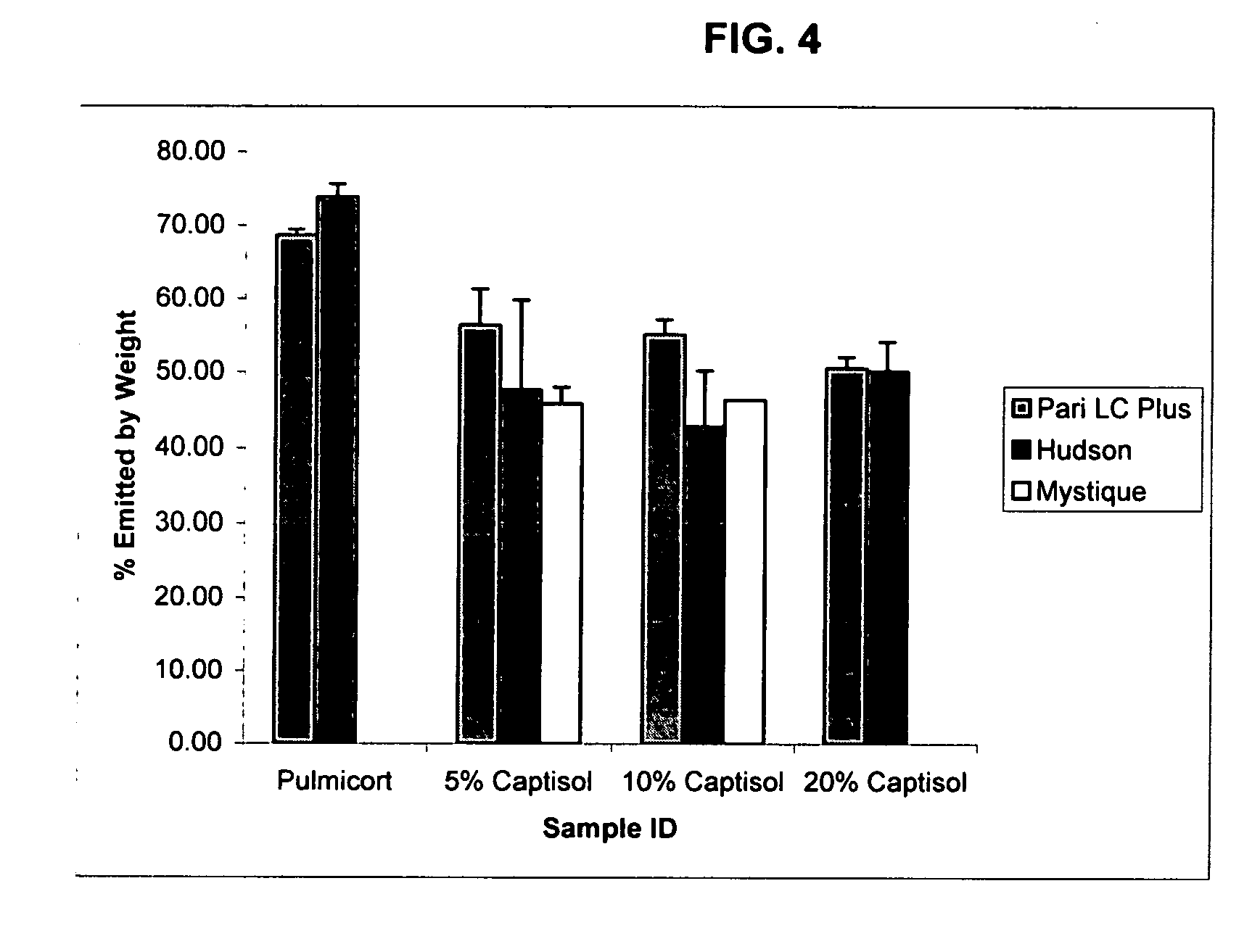
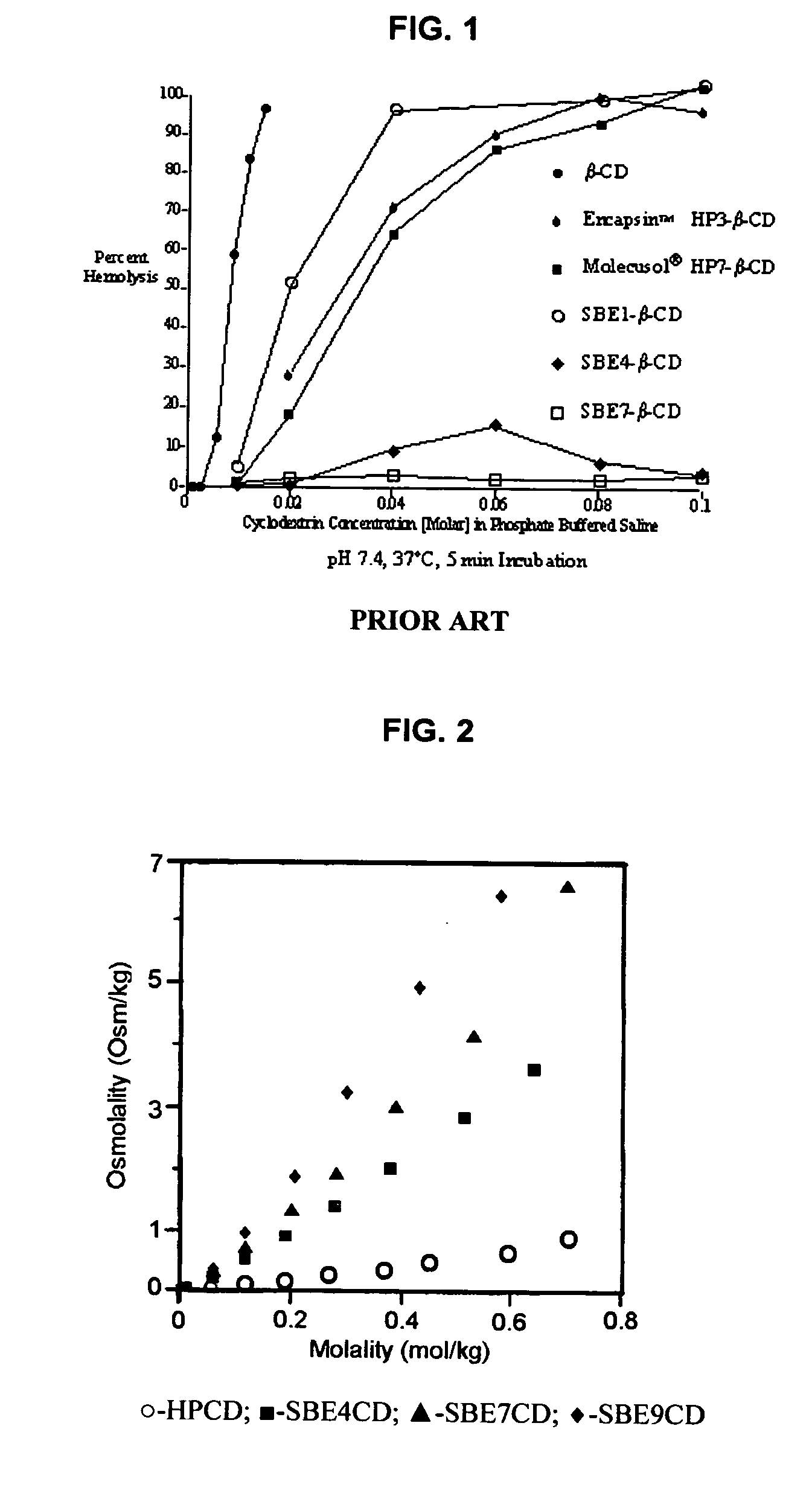
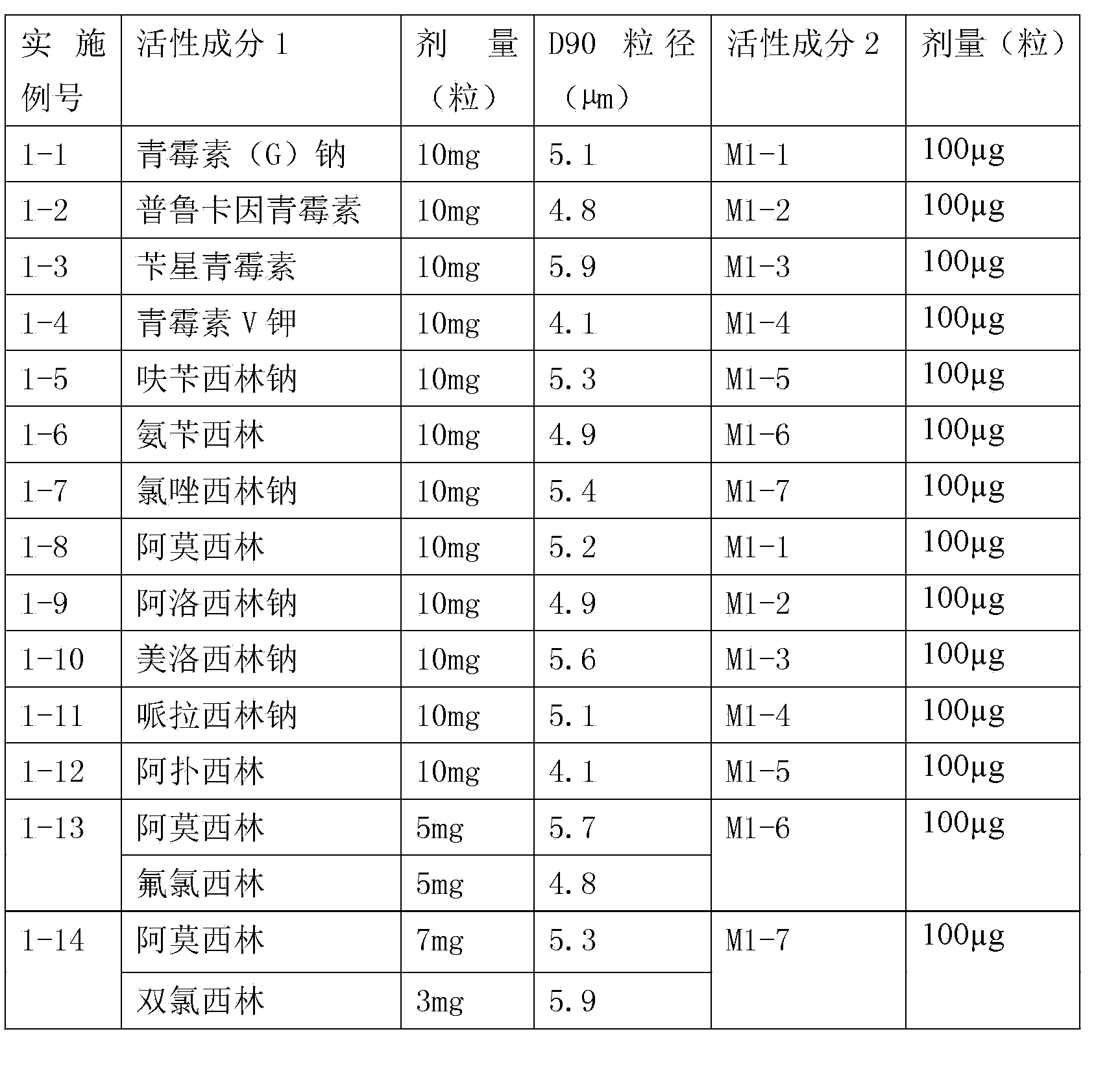
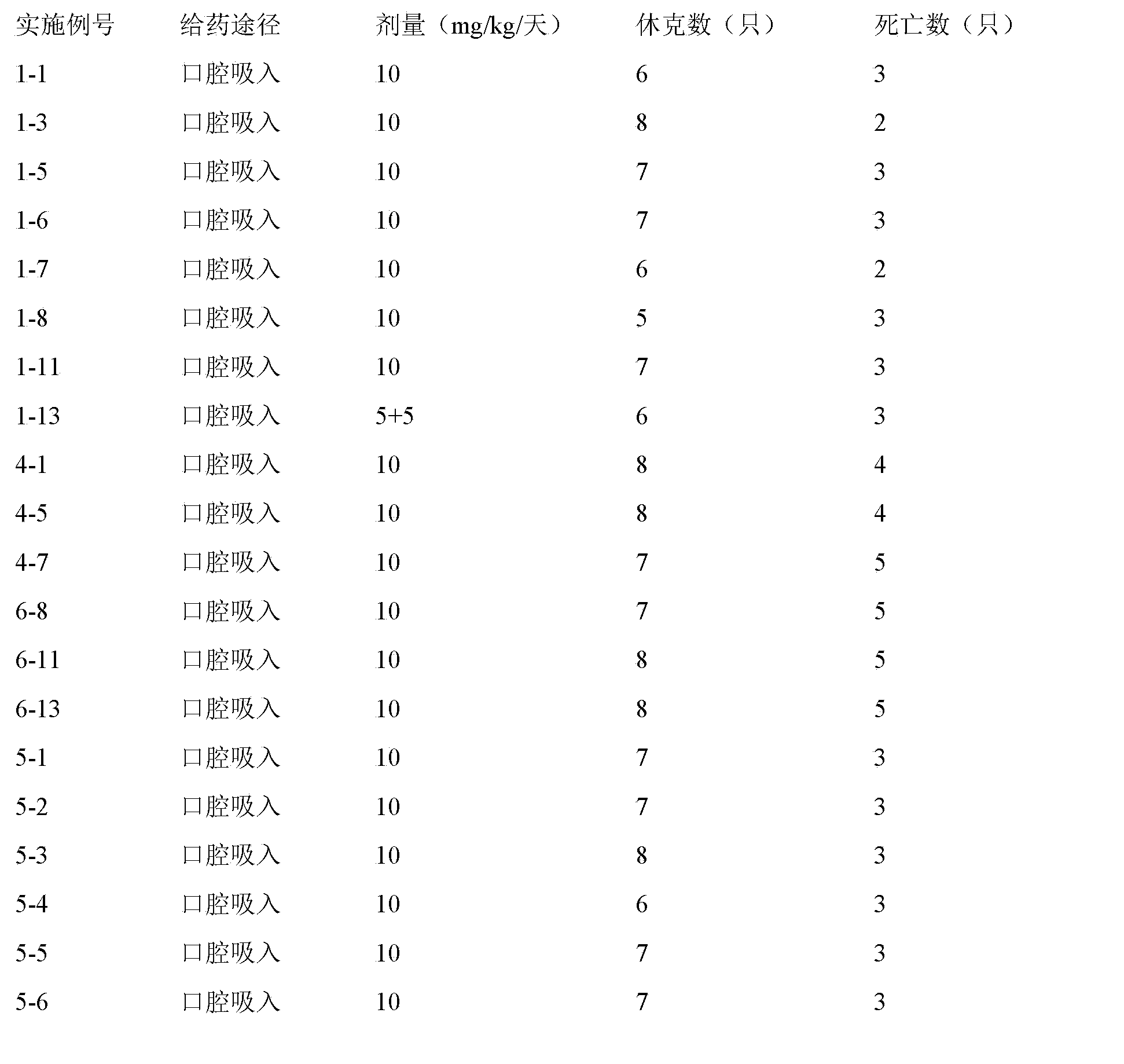
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
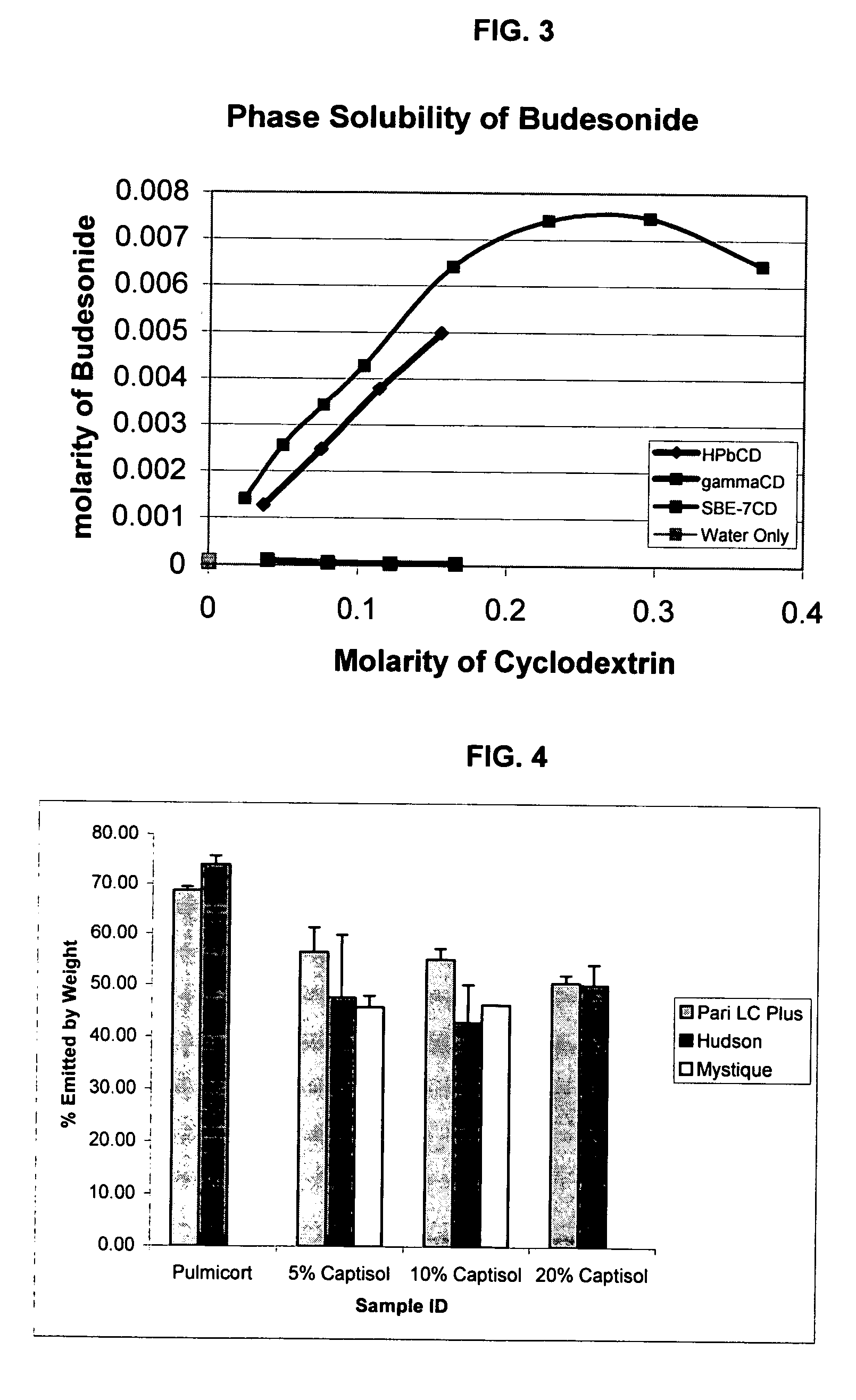
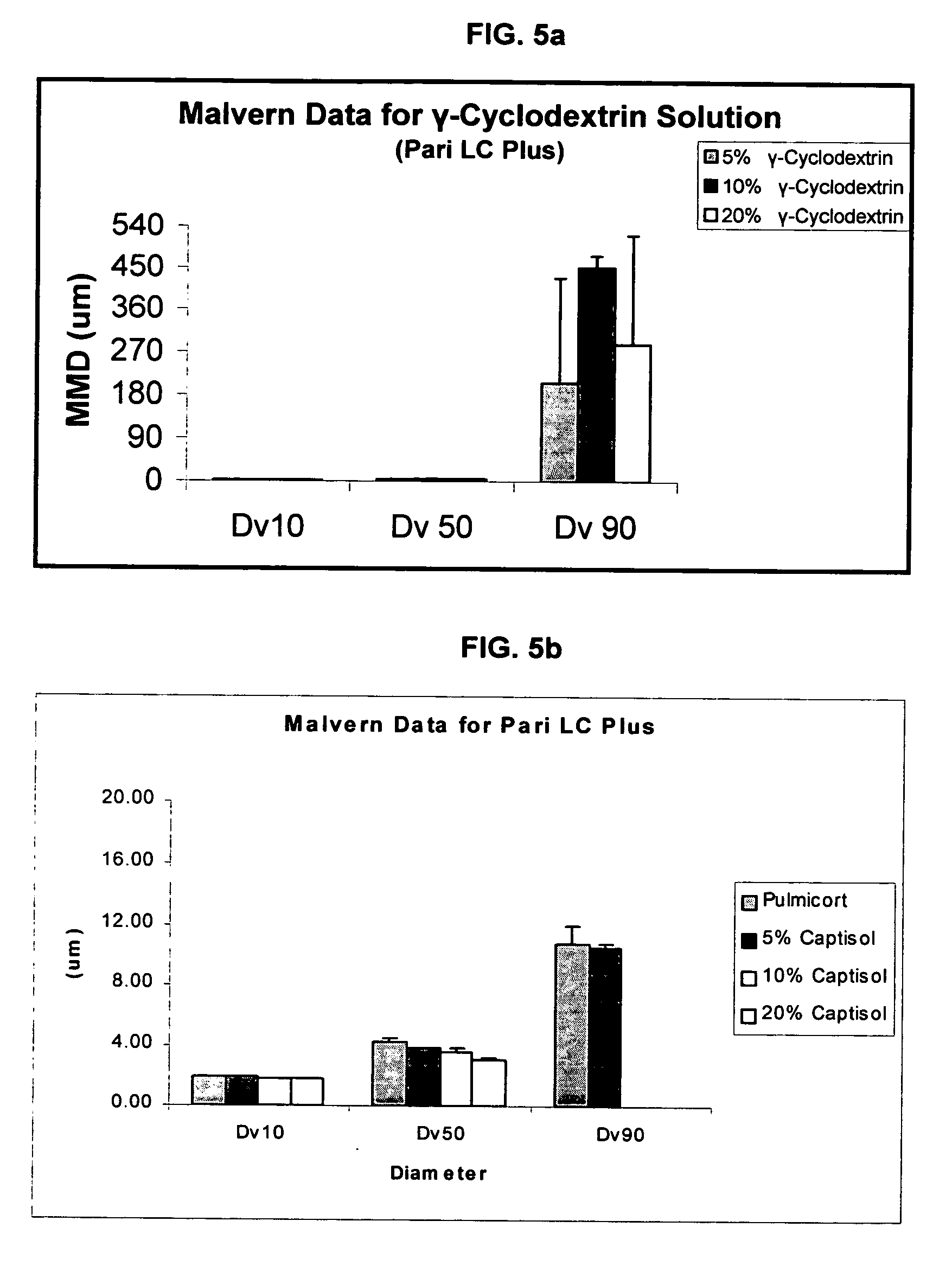
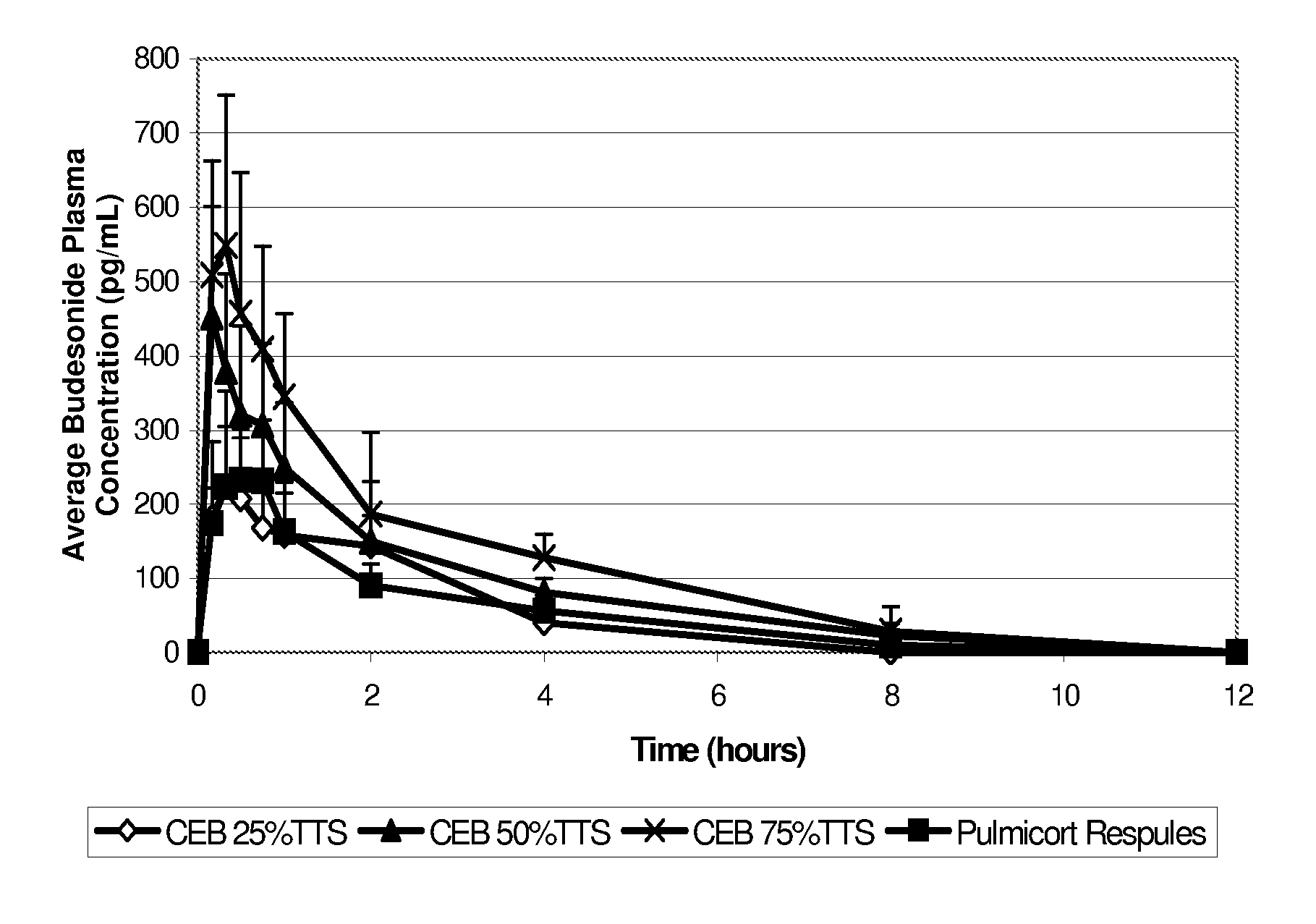
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid
InactiveUS20070202054A1Improve solubilityImprove stabilityBiocidePowder deliveryNebulizerCyclodextrin
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMA INC
Injection-grade ambroxol hydrochloride and solution for inhalation of injection-grade ambroxol hydrochloride
InactiveCN102924302AImprove stabilityQuality improvementOrganic active ingredientsOrganic compound preparationInhalationAqueous ethanol
The invention relates to a method for refining injection-grade ambroxol hydrochloride. The method is characterized by comprising the steps of adding oral-taking-grade ambroxol hydrochloride with the proportion smaller than or equal to 20:1 into ethanol water solution with the volume ratio of 5-20%; performing heating to completely dissolve the oral-taking-grade ambroxol hydrochloride; stopping heating and performing cooling to seed out ambroxol hydrochloride; and filtering solvent to obtain crystals and drying the crystals to obtain the injection-grade ambroxol hydrochloride. The invention further relates to ambroxol hydrochloride solution for inhalation prepared by utilizing the prepared injection-grade ambroxol hydrochloride as the raw material. The atomization inhalation solution comprises the ambroxol hydrochloride, a stabilizer, a pH conditioning agent and an osmotic pressure conditioning agent. By means of the scientific preparation adopting the carrier, fewest degradation products of the atomization inhalation preparation can be produced under the high temperature of 121 DEG C for 15 minutes, the atomization inhalation preparation is stable, and the service life of the atomization inhalation preparation is prolonged to 5 years.
Owner:HC SYNTHETIC PHARMA CO LTD
Pharmaceutical compositions comprising apomorphine for pulmonary inhalation
InactiveUS20060178394A1Improve performanceRapid blood levelBiocidePowder deliveryPulmonary inhalationSexual dysfunction
The present invention relates to inhalable formulations of apomorphine or its pharmaceutically acceptable salts or esters for use in treating sexual dysfunction. The present invention also relates to methods for preparing the apomorphine formulations as well as to methods for treatment of sexual dysfunction using said formulations and inhalers including said formulations. The present invention further relates to the use of apomorphine in the manufacture of a medicament for treating sexual dysfunction.
Owner:VECTURA LTD
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveCN1921834AIncreased dosing rateShorten treatment timePowder deliveryDispersion deliveryNebulizerCyclodextrin
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX INC
Processes for taste-masking of inhaled formulations
InactiveUS20080138397A1Minimizing bitter tasteMinimizing cough creationBiocideDispersion deliveryPulmonary inhalationThroat irritation
The present invention provides novel processes and methodologies to minimize the bitter or otherwise unpleasant taste, to minimize the tendency to stimulate the cough reflex, or to minimize oropharyngeal deposition of medically-active compounds administered by the pulmonary / inhalation route and to deliver hydroxychloroquine (HCQ) either singularly or in combination with an antimalarial and aminoquinolone by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the bitter or otherwise unpleasant taste of HCQ or any potential to stimulate the cough reflex, and to deliver a dopaminergic compound or its prodrug, including ABT-431 by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate the cough reflex, and to deliver a lantibiotic, including duramycin by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate throat irritation.
Owner:ARADIGM
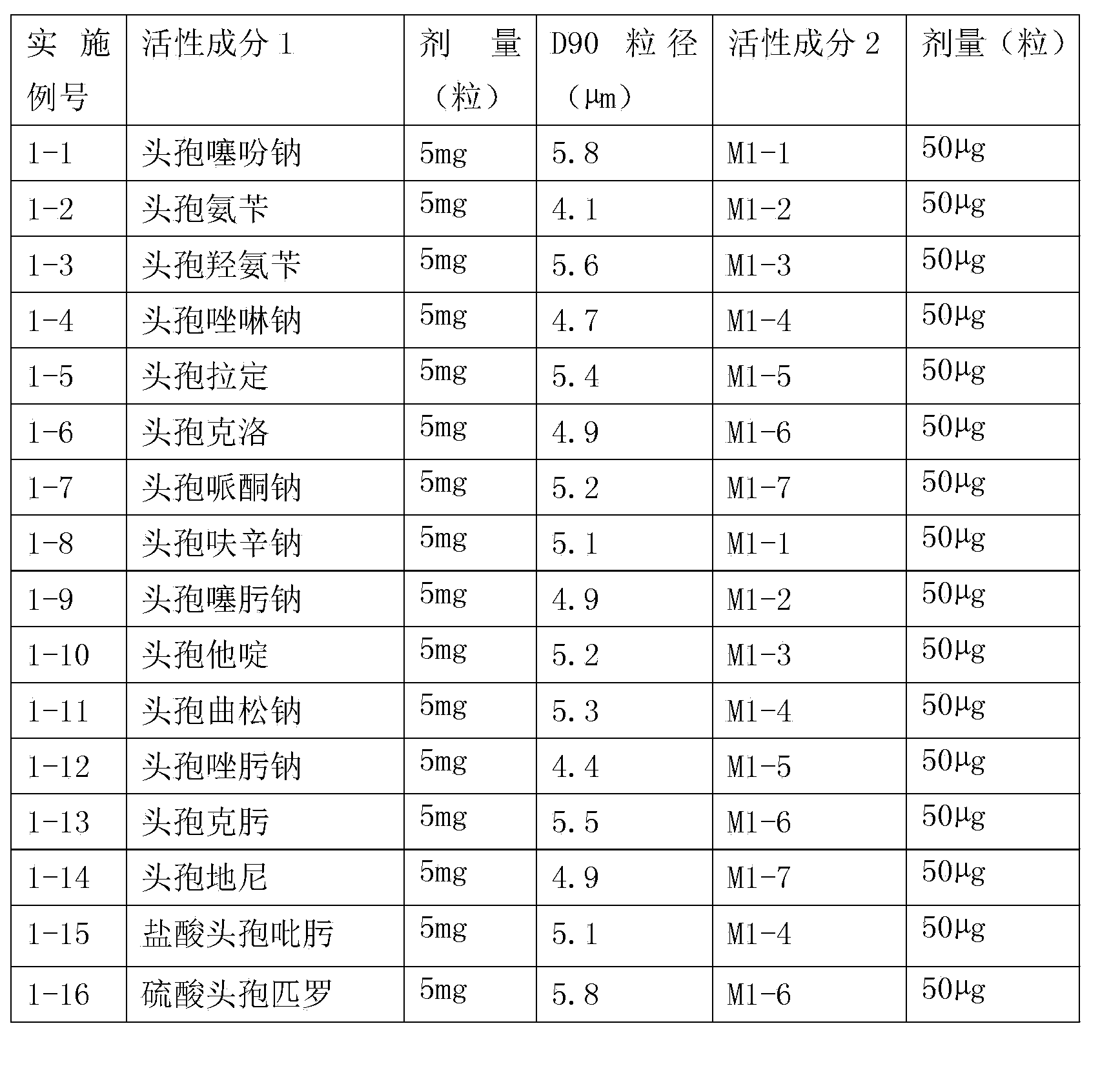
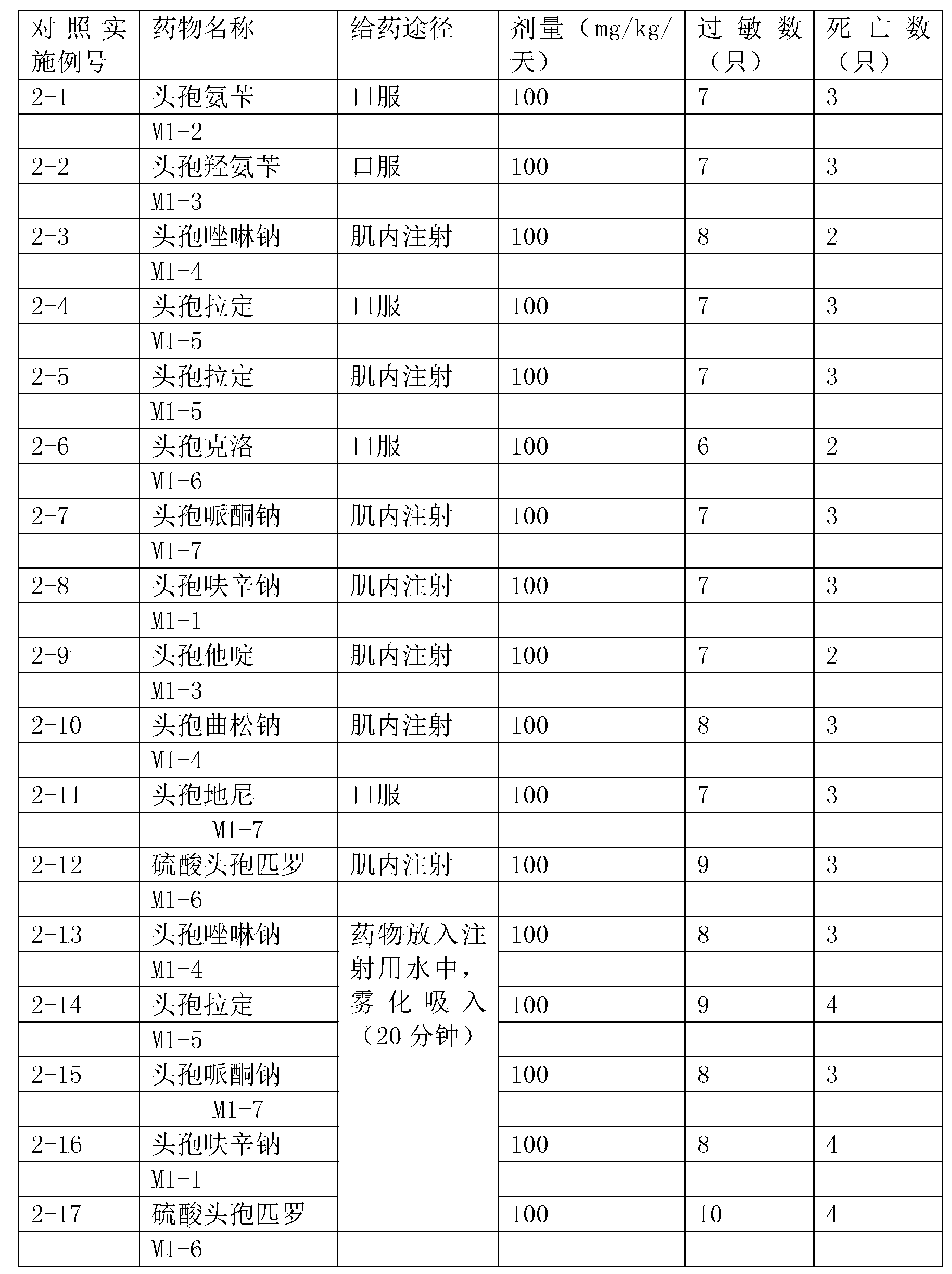
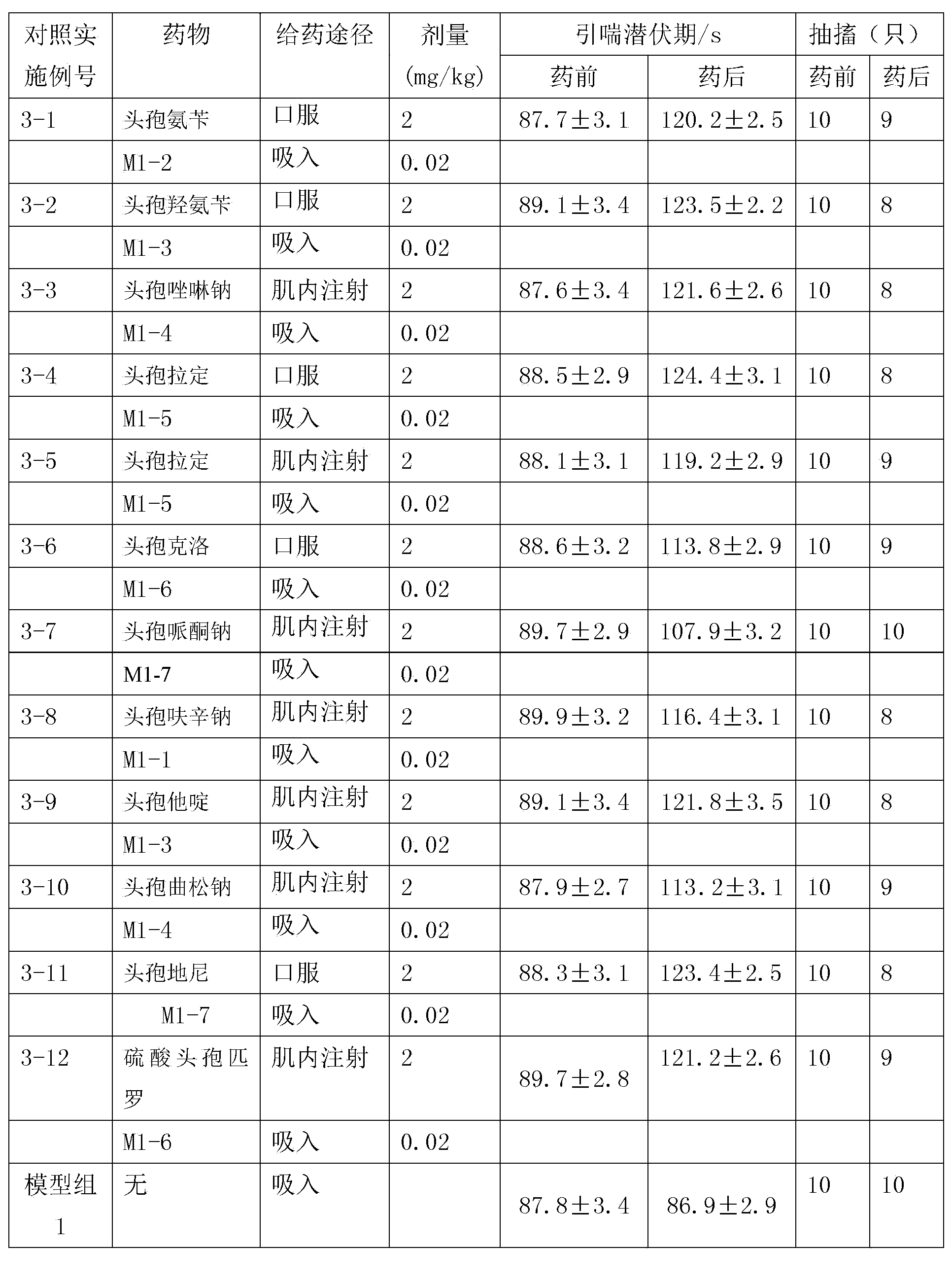
Inhalation preparation containing cephalosporin antibiotic
The invention relates to an inhalation preparation containing cephalosporin antibiotic and applications thereof. The cephalosporin antibiotic is made into an inhalation dosage form, and thus the preparation can treat pulmonary bacterial infection with less dosage and has a certain curative effect on asthma and chronic obstructive pneumonia.
Owner:TIANJIN JINYAO GRP
Compound inhalation preparation containing cephalosporin antibiotic and glucocorticoid
The invention relates to a compound inhalation preparation containing cephalosporin antibiotic and glucocorticoid and applications thereof. The cephalosporin antibiotic is made into an inhalation dosage form, so that the preparation can treat pulmonary bacterial infection with less dosage form and has a certain curative effect on asthma and chronic obstructive pneumonia at the same time.
Owner:TIANJIN JINYAO GRP
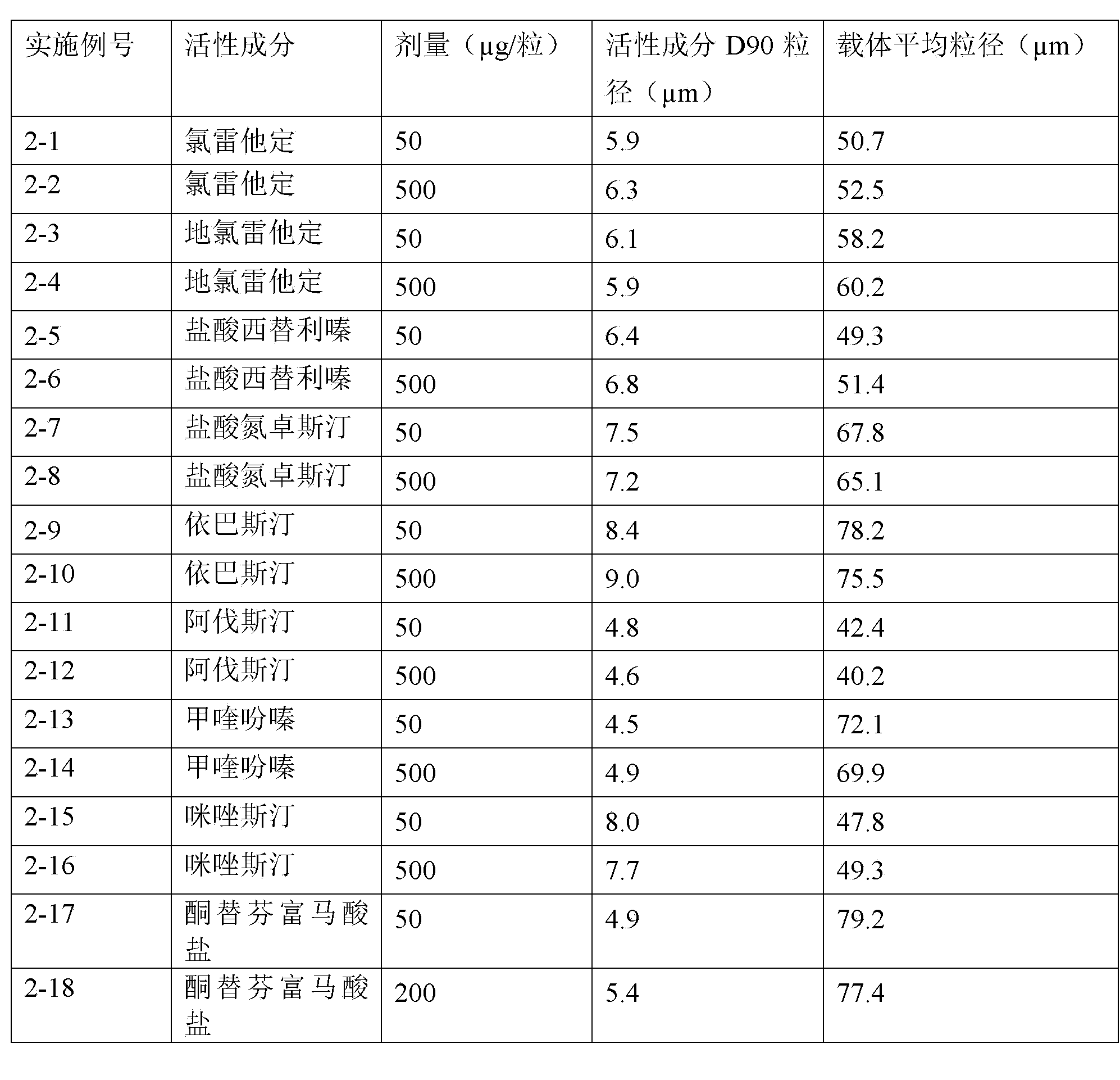
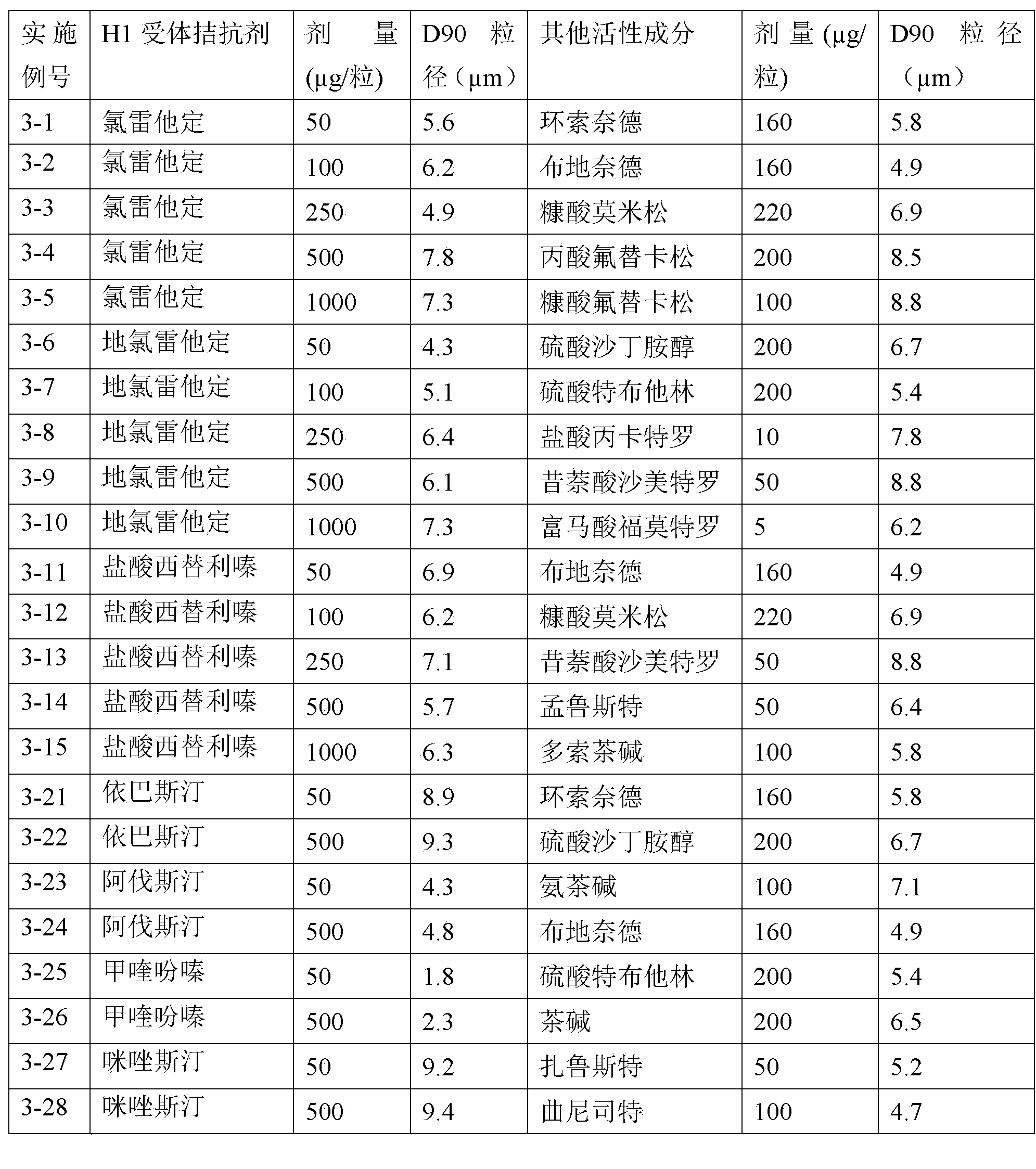
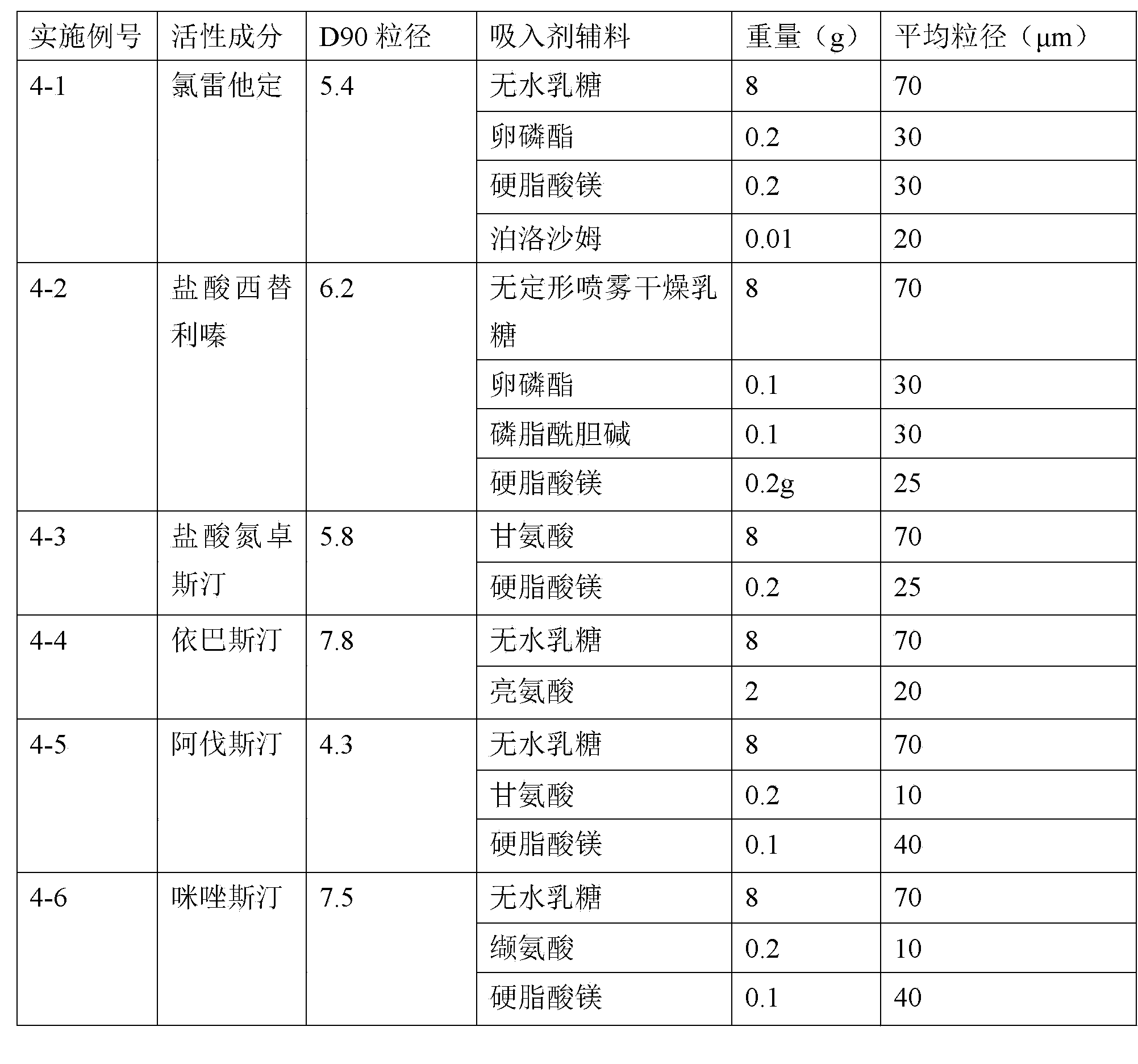
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Inhalable Formulations
InactiveCN102811715ALow densityImprove mechanical propertiesOrganic active ingredientsPowder deliveryInhalable particlesActive agent
Respirable inhalable particles comprising active agent(s) and excipients in at least a partially crystalline form and their uses thereof.
Owner:THE UNIV OF SYDNEY
Pharmaceutical composition for treating viral infection of respiratory system
InactiveCN112386595AImprove securityReduce doseOrganic active ingredientsDispersion deliveryPharmaceutical drugInhalation preparations
The invention provides a pharmaceutical composition for treating viral infection of respiratory system. The pharmaceutical composition contains chloroquine and a sputum-removing drug. The sputum-removing drug is selected from at least one of acetylcysteine, ambroxol or ammonium chloride. The invention provides a preparation method of the pharmaceutical composition for preventing and treating diseases caused by virus infection of a human respiratory system. The medicine composition of the chloroquine and sputum-removing drug is prepared into a spray, an aerosol or an inhalation preparation forpreventing and treating the diseases caused by the virus infection of the human respiratory system.
Owner:徐静 +1
Ambroxol hydrochloride solution for inhalation
The invention relates to an ambroxol hydrochloride solution for inhalation and a preparation method thereof. Ambroxol hydrochloride is used as an active ingredient. The ambroxol hydrochloride solution belongs to a sterile inhalation preparation capable of being used for treating diseases such as bronchial asthma, chronic bronchitis, emphysema, pulmonary heart diseases, pulmonary infection and COPD (chronic obstructive pulmonary diseases).
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Application of houttuynia cordata and honeysuckle injection used for preparing rectal administration preparation and atomizing inhalation preparation
InactiveCN104382841AImprove bioavailabilityGood treatment effectDigestive systemPharmaceutical delivery mechanismTreatment effectCurative effect
The invention belongs to the field of pharmaceutical preparations, and particularly relates to novel application of a houttuynia cordata and honeysuckle injection used for preparing a rectal administration preparation and an atomizing inhalation preparation. The houttuynia cordata and honeysuckle injection comprises the following raw materials: 3500-4500 parts of houttuynia cordata, and 1500-2500 parts of honeysuckle. The rectal administration preparation and the atomizing inhalation preparation have the characteristics that the rectal administration preparation and the atomizing inhalation preparation are quick in absorption, avoid first pass effect, are convenient to operate, have no pain and trauma, are easy to be accepted particularly by child patients, directly act on pathological changes tissue towards part diseases, are quick in effect, and improve bioavailability of the medicine, and has outstanding curative effect for infectious diseases.
Owner:王锋
Compound inhalation preparation containing antifungal drug
The invention relates to the components and applications of a compound inhalation preparation containing an antifungal drug. When a trace amount of an antifungal drug is compounded with a drug for treating asthma and chronic obstructive pneumonia, a good curative effect will be generated; and the curative effect is more prominent, when the antifungal drug is compounded with glucocorticoid and an anti-cholinergic agent.
Owner:TIANJIN JINYAO GRP
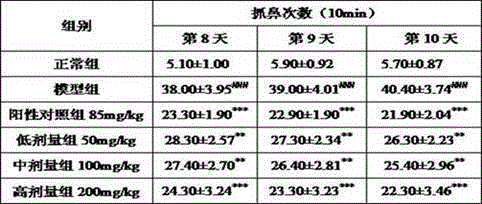
Preparation method of guinea pig airway allergic asthma model
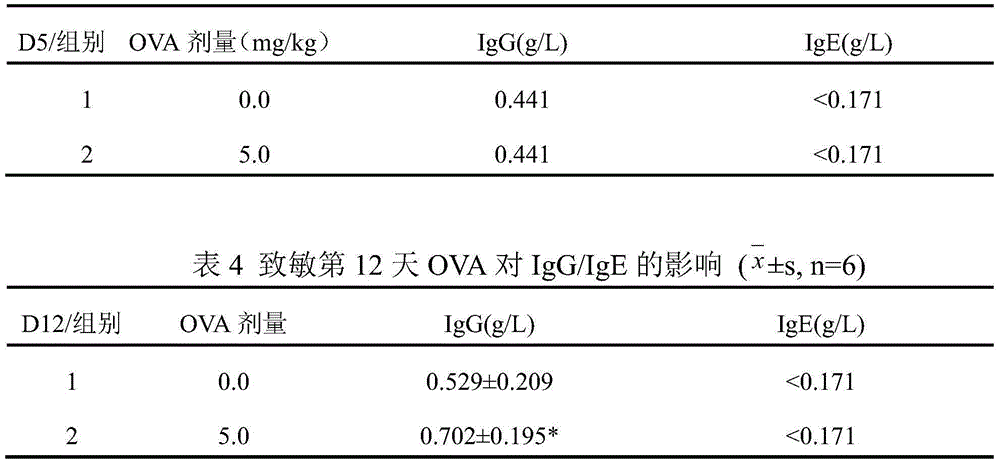
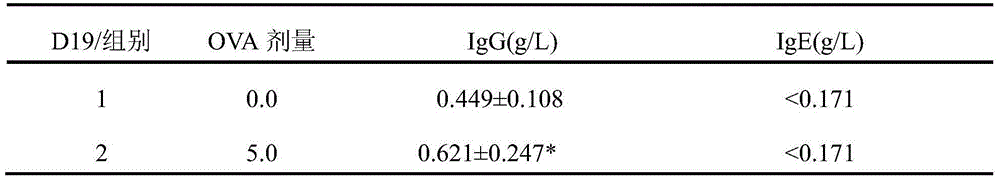
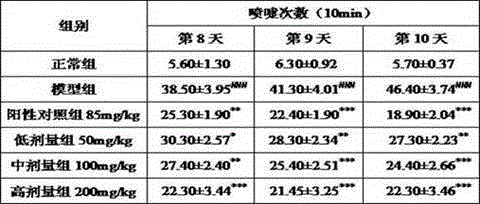
The invention discloses a preparation method of a guinea pig airway allergic asthma model. The preparation method comprises the steps as follows: (1) injecting an OVA (ovalbumin) normal saline solution into a guinea pig abdominal cavity once every other day, and injecting the solution two to four times totally to enable a guinea pig to be allergic; (2) on 12th-15th days after the last sensitization in Step (1), enabling the guinea pig to inhale the atomized OVA normal saline solution through the mouth and the nose so as to arouse airway allergic asthma. Compared with existing methods, the preparation method has the advantages that the aerosol inhalation delivery is simulated, an inhalation preparation for nasal delivery is simulated, the target organ is sole, the pertinence is high, and common guinea pig intravenous injection allergy arousing tests cannot predict whether the allergy is aroused due to inhalation of a drug through the respiratory tract or not. Experiments prove that the preparation method is good in allergic effect, the OVA normal saline solution which is only inhaled and atomized through the mouth and the nose serves as a way to arouse the allergy, and the guinea pig airway allergic asthma model is successfully established.
Owner:SHANGHAI INNOSTAR BIO TECH +1
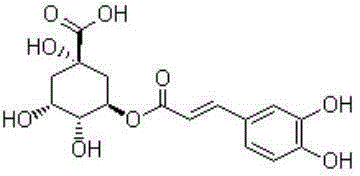
Application of chlorogenic acid to preparing medicine for treating allergic rhinitis
The invention discloses application of chlorogenic acid to preparing medicine for treating allergic rhinitis. The chlorogenic acid can be used for preparing compositions in diversified forms. The compositions include oral, injection and lung inhalation preparations and particularly include injection, oral liquid, tablets, capsules, granules, aerosol, powder aerosol and spray.
Owner:洪铁 +1
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
ActiveCN1976679AIncreased dosing rateShorten treatment timePowder deliveryDispersion deliveryNebulizerEther
An inhalable formulation containing SEA-gamma-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-gamma-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Lopinavir inhalation aerosol and preparation method thereof
ActiveCN111265499AImprove stabilityAccurate dosePowder deliveryOrganic active ingredientsPulmonary infectionActive agent
The invention belongs to the technical field of medicines, and discloses a lopinavir inhalation aerosol and a preparation method thereof. The lopinavir inhalation aerosol is composed of lopinavir serving as an active ingredient, a propellant, a cosolvent, a surfactant and the like according to a certain ratio . The aerosol inhalant is administrated through the oral cavity and directly acts on thelung to achieve targeted administration. The inhalation preparation disclosed by the invention can target a focus, is accurate in dosage, takes effect rapidly, can quickly improve the pulmonary infection condition, is beneficial to improve the adaptability of an infected person, and avoids gastrointestinal tract absorption so as to reduce side effects on the gastrointestinal tracts.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Compound inhalation preparation containing penicillin antibiotic and glucocorticoid
The invention relates to a compound inhalation preparation containing penicillin antibiotic and glucocorticoid and applications thereof. The preparation is capable of treating pulmonary bacterial infection with less dosage and has a certain curative effect on asthma and chronic obstructive pneumonia. The preparation is made into an dry powder inhalation dosage form, after a long period of preservation, the content of allergic matters namely penicilloic acid and penicillenic acid is not increased, so that the happening rate of allergy is largely reduced, when the preparation is used to treat asthma caused by non-microbial infection and chronic obstructive pneumonia.
Owner:TIANJIN JINYAO GRP
Composite drug-loaded extracellular vesicle inhalation preparation as well as preparation method and application thereof
PendingCN112870181ALower doseSmall toxicityOrganic active ingredientsDispersion deliveryExtracellular vesicleLung tumours
According to the composite drug-loaded extracellular vesicle inhalation preparation and the preparation method and application thereof, the extracellular vesicle inhalation preparation is used for treating lung tumors in an oral inhalation administration mode, the residence time of treatment drugs in a body can be effectively prolonged, distribution of the drugs in other non-target tissues and organs in the body is reduced. The toxic and side effects of the medicine are reduced while the treatment effect is enhanced, and the medicine shows a good treatment effect of remarkably inhibiting the growth of drug-resistant lung tumors in an animal model.
Owner:GUANGDONG UNIV OF TECH
Kits for manufacturing inhalable formulations of medicinal cannabis compounds for aerosol devices, apparatuses, and methods for making and using the same
ActiveUS20200288786A1Save a lot of timeEasy to fillRespiratorsPowder deliveryAerosol sprayBiochemical engineering
Kits enabling an end user to efficiently compound inhalable formulations containing medicinal compounds, for use in in aerosol device (e-cigarette; personal vaporizer). Kits include a reusable storage device, a filling station, at least one predetermined amount aerosol precursor in a container and at least one empty cartomizer (cartridge) for use in an aerosol delivery device. Optionally, kits may also include a predetermined amount of extract containing a known amount of medicinal compound, enabling the production of an amount an inhalable formulation. Optionally, kits may contain amounts of inputs that enable the refilling / reuse of cartridges.
Owner:NIEBLING AVRAM
Compound inhalation preparation containing penicillin antibiotic
The invention relates to a compound inhalation preparation containing penicillin antibiotic and applications thereof. The preparation in an inhalation dosage form is capable of treating pulmonary bacterial infection with less dosage and has a certain curative effect on asthma and chronic obstructive pneumonia. The preparation is made into an dry powder inhalation dosage form, after a long period of preservation, the content of allergic matters namely penicilloic acid and penicillenic acid is not increased, so that the happening rate of allergy is largely reduced, when the preparation is used to treat asthma caused by non-microbial infection and chronic obstructive pneumonia.
Owner:TIANJIN JINYAO GRP
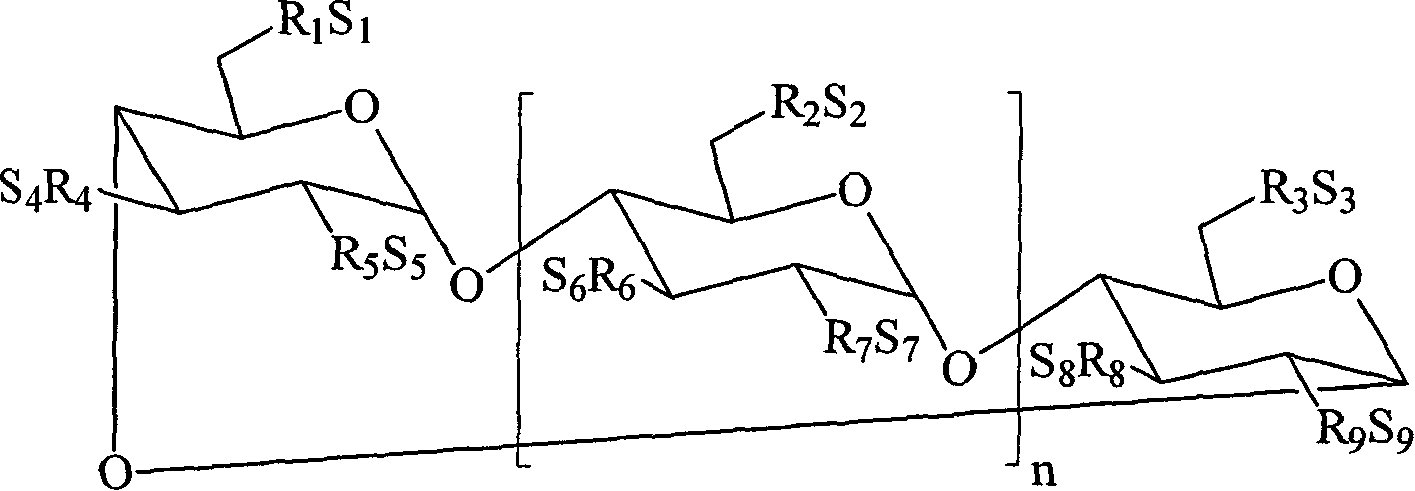
Novel inhalation preparation
ActiveCN106551919AParticle size unchangedReduce the overall heightPowder deliveryOrganic active ingredientsFreeze-dryingChemical stability
The invention relates to a novel inhalation preparation, in particular to a preparation suitable for inhalation drug administration of children ranging from 0 to 12 years. After medicine difficult to be dissolved in water and auxiliary materials are mixed, a wet grinding method is adopted for smashing, mixed suspension liquid containing particles with the particle size appropriate is obtained, the particle size is 0.1-7 microns, and preferentially, the particle size is 0.1-5 microns; and further, the preferential particle size is 0.1-3 microns. Solid powder is obtained through a freeze-drying method. Sealing is performed for obtaining the novel inhalation; and before use, the novel inhalation preparation is mixed with liquid suitable for atomization, re-dispersion is performed, and the mixed suspension liquid capable of being used for atomizing inhalation of children is obtained. The preparation is stored and transported in the form of the solid powder, the problem that when a mixed suspension liquid form is adopted, the physical stability and the chemical stability are poor is avoided, the medicine particle size hardly changes, impurity increase is little, and the content hardly changes. Compared with inhalation powder aerosol, the novel inhalation preparation is especially suitable for atomizing medicine administration of children, medicine smaller in particle size can be adopted, and even though under the situations that breathing of an infant is slight and the effective portion deposition rate is low, an ideal medicine effect can also be achieved.
Owner:北京天衡药物研究院有限公司
Inhalation preparation containing calcitriol and budesonide and preparation method thereof
InactiveCN102247385ALong-term use aloneLower serum IgEOrganic active ingredientsPharmaceutical delivery mechanismDiseaseActive component
The invention discloses an inhalation preparation containing calcitriol and budesonide and a preparation method thereof. The inhalation preparation containing calcitriol and budesonide comprises calcitriol and budesonide as active components and one or more pharmaceutic adjuvants suitable for inhaled drugs, wherein, the weight ratio of calcitriol to budesonide is 100-800:1. The inhalation preparation containing calcitriol and budesonide can be used for treating bronchial diseases in humans or mammals.
Owner:TIANJIN JINYAO GRP
Inhalation powder mist preparation for preventing and treating respiratory tract infectious diseases
PendingCN113244262AEffective Lung Targeted DrugsAntibacterial agentsPowder deliveryPulmonary infectionDirect targeting
The invention relates to an inhalation powder mist preparation for preventing and treating respiratory tract infectious diseases. Specially, the invention relates to an inhalation preparation for preventing and treating respiratory tract infectious diseases, in particular to an inhalation powder mist preparation. The inhalation powder mist preparation comprises iodine molecules as an active substance and / or a pharmaceutically acceptable carrier. The medicine is inhaled into the respiratory tract or the lung through powder mist for preventing and treating infectious diseases of the respiratory tract and the lung, and has the advantages of direct targeting action on infected parts, quick response, small dosage and small side effect. Various pathogens in the respiratory tract can be rapidly killed, and the purposes of reducing infectivity, relieving pain of a patient, shortening the rehabilitation period, improving the cure rate, reducing complications and reducing the death rate are achieved.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
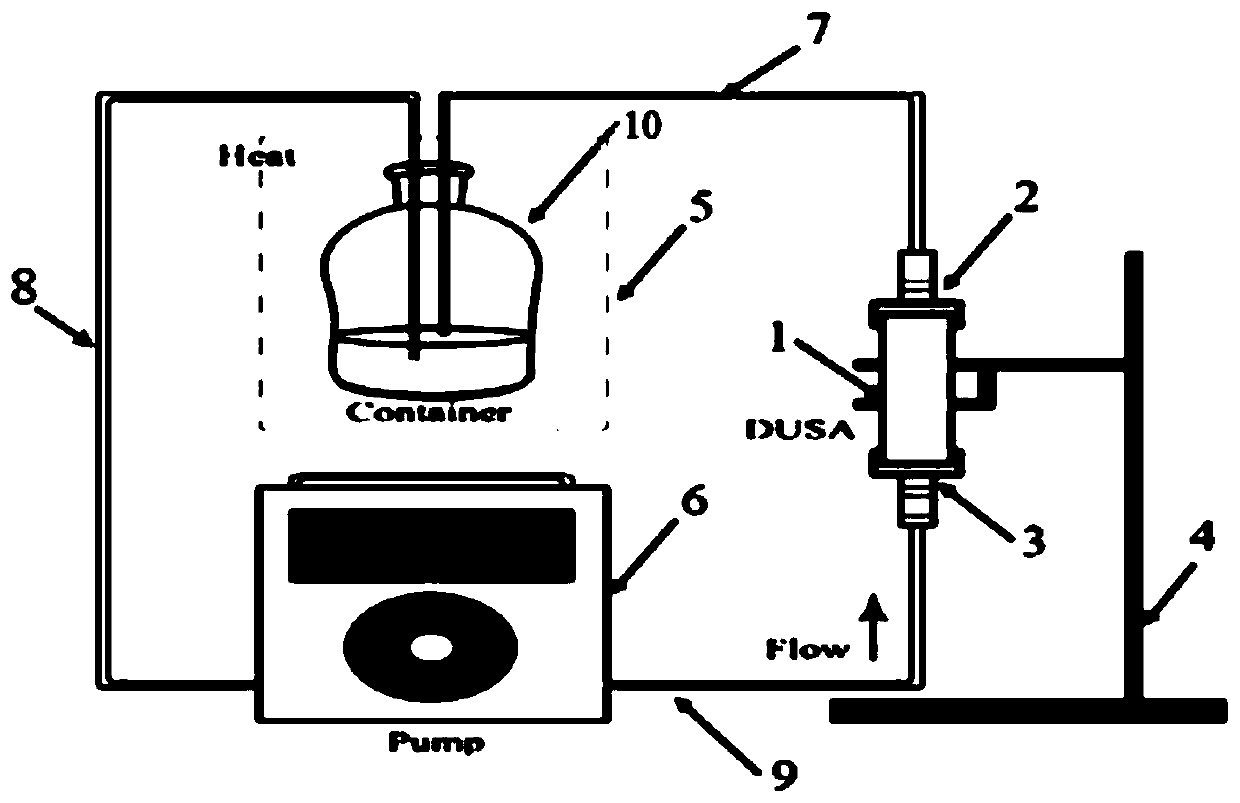
Device and method for measuring dissolution of inhalation preparation
PendingCN109827875AEasy to applyEasy to operateComponent separationSurface/boundary effectDissolutionInhalation preparations
The invention relates to a device for measuring dissolution of an inhalation preparation. The device comprises a DUSA pipe, a dissolution medium pump and a dissolution medium container, wherein a flowmeter connecting head and a vacuum pump connecting head are respectively mounted at both ends of the DUSA pipe; the flowmeter connecting head is connected with the dissolution medium container througha first pipeline; the dissolution medium container is connected with one end of the dissolution medium pump through a second pipeline; and the vacuum pump connecting head is connected with the otherend of the dissolution medium pump through a third pipeline. The invention further relates to a method for measuring the dissolution of the inhalation preparation by adopting the above device. The dissolution device of the inhalation preparation makes up the vacancy of the existing dissolution device, the dissolution method is simple to operate, simulates the lung dissolution environment, is convenient to sample, adopts closed circulation dissolution in the whole dissolution process, is simple to apply and convenient to operate, and less in use of the dissolution medium in the whole dissolution process, and can select multiple operation parameters according to actual needs, so that the feasibility of a dissolution test of the inhalation preparation and the reliability of a dissolution result are guaranteed.
Owner:SHANGHAI INST FOR FOOD & DRUG CONTROL
Application of indometacin to resistance to coronavirus infections
The invention relates to application of indometacin to resistance to coronavirus infections, and provides new application of the indometacin to inhibition from the SARS coronavirus and a novel preparation of the indometacin. The novel preparation is an inhalation preparation. The indometacin can obviously inhibit the SARS coronavirus, and thus is expected to be a medicine for treating diseases caused by the SARS coronavirus.
Owner:徐天宏
Chloroquine phosphate inhalation aerosol and preparation method thereof
InactiveCN111110634AImprove stabilityAccurate doseOrganic active ingredientsDispersion deliveryInhalationPhosphoric acid
The invention belongs to the technical field of medicine, and discloses a chloroquine phosphate inhalation aerosol and a preparation method thereof. The chloroquine phosphate inhalation aerosol is composed of active ingredient chloroquine phosphate and a certain proportion of a propellant, a flavoring agent, a pH regulator and water for injection. The aerosol inhaler is administered through mouthsand directly acts on lungs to realize targeted drug delivery. The inhalation preparation provided by the invention can target lesion, has an accurate dosage and a fast effect, can quickly improve theinfection conditions of the lungs, facilitates improving the adaptability of infected people, and avoids absorption through gastrointestinal tracts to reduce gastrointestinal side effects.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Application of earthworm injection in atomization and rectal administration and preparation method thereof
InactiveCN109288877AMeet the requirements of physical and chemical propertiesNon-irritatingPowder deliverySpray deliveryDiseaseIrritation
Owner:广东新峰药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com