Device and method for measuring dissolution of inhalation preparation
A technology for inhalation preparations and dissolution rate, which is applied in the field of devices for measuring the dissolution rate of inhalation preparations, can solve problems such as the vacancy of dissolution devices, and achieve the effects of convenient operation, convenient sampling, and simple application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
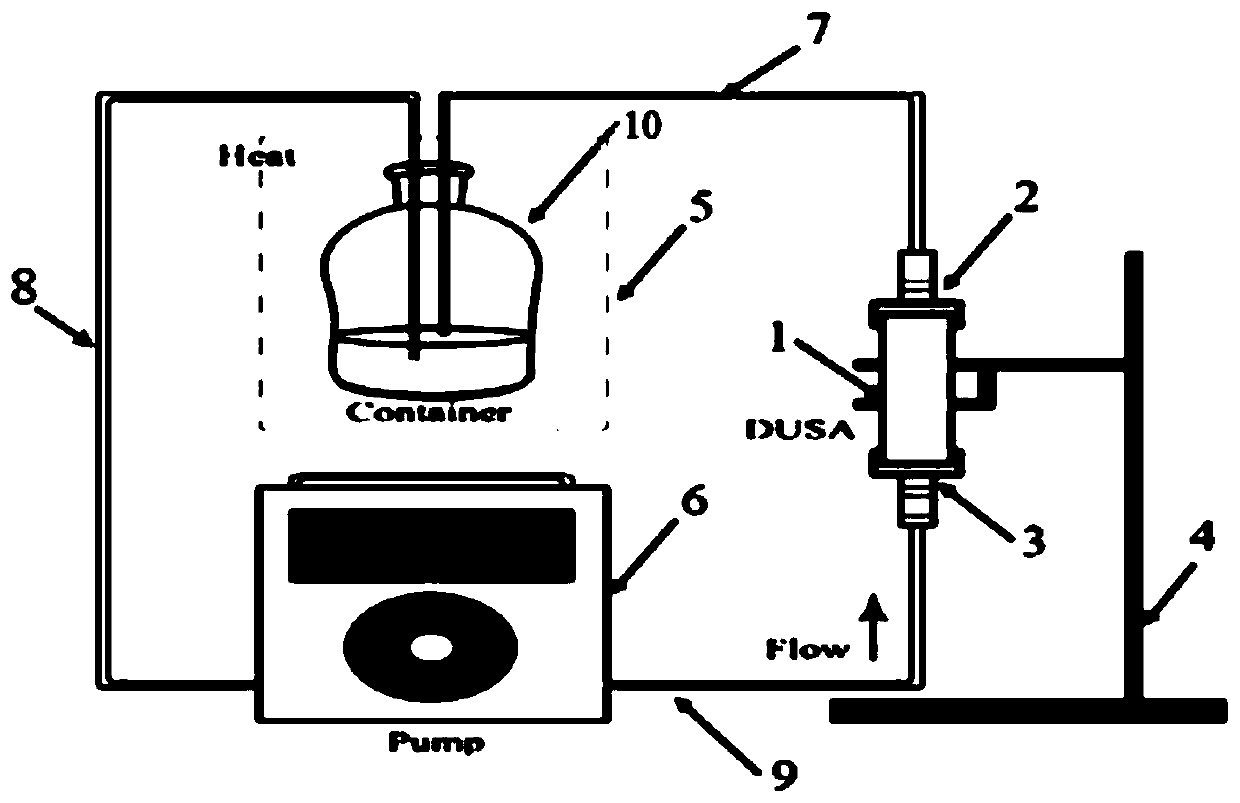
[0051] This embodiment relates to a device for determining the dissolution rate of inhalation preparations, such as Figure 1~4 As shown, the above-mentioned device includes a DUSA tube 1 for sample collection, a flowmeter connector 2 and a vacuum pump connector 3 installed on both ends of the DUSA tube 1, and a fixed frame 4 for fixing the DUSA tube 1 for storing the dissolution medium The dissolution medium container 10, the heat preservation device 5 for keeping the dissolution medium warm, the dissolution medium pump 6 for driving the dissolution medium to circulate, the flowmeter connector 2, the vacuum pump connector 3 and the dissolution medium pump 6 are respectively connected with First pipeline 7 , second pipeline 8 and third pipeline 9 .
[0052] In a preferred embodiment, the heat preservation device 5 is a water bath. The first pipeline 7, the second pipeline 8 and the third pipeline 9 are all hoses. The dissolution medium pump 6 is a peristaltic pump. The fixi...
Embodiment 2
[0057] This embodiment is a method for measuring the dissolution rate of an inhalation preparation using the device in Example 1, which includes the following steps:
[0058] Step 1. Open the heat preservation device, adjust the dissolution temperature, and place the dissolution medium container in the heat preservation device for heat preservation, wherein the dissolution medium is 0.2mol / L phosphate buffer (pH=7.4);
[0059] Step 2. Take an appropriate amount of inhalation preparation sample, place the DUSA tube in the delivery dose uniformity test device for sampling, after the sampling is completed, remove the DUSA tube from the delivery dose uniformity test device, install the vacuum pump connector, and install it on a fixed On the rack, fill glass beads in the DUSA tube, cover the glass beads with round filter paper, and install the flowmeter connector;
[0060] Step 3: The flow meter connector is connected to the dissolution medium container through the first pipeline; ...
Embodiment 3
[0064] This example is to apply the device described in Example 1 and the method described in Example 2 to carry out the dissolution test of inhalation powder aerosol.
[0065] (1) Purpose: To study the dissolution test method of solid preparations for pulmonary administration, formulate a reasonable measurement method, and compare the in vitro release behavior of domestic and foreign powder aerosols in the market, so as to advance the quality standard of powder aerosols lay the foundation.
[0066] (2) Test object
[0067] There are 4 batches of budesonide powder and salmeterol-ticasone powder, see Table 1 for details.
[0068] Table 1 collects sample information
[0069]
[0070] (3) Progress of dissolution test protocol
[0071] Take an appropriate amount of powder aerosol sample, according to the method under item 0111 of the Four General Rules of the Chinese Pharmacopoeia 2015 Edition, use the delivery dose uniformity measurement device, collect the powder aerosol p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com