Composite drug-loaded extracellular vesicle inhalation preparation as well as preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
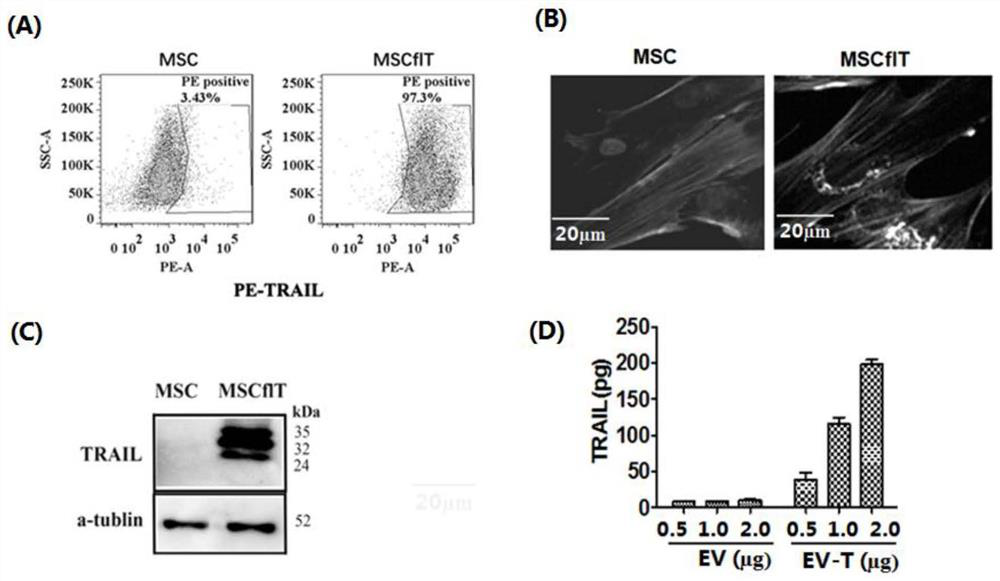
[0032] Example 1 Preparation and Characterization of TRAIL Genetically Modified MSC Stem Cells
[0033] 1. TRAIL genetic engineering modification of MSC cells: Subculture P3-P5 umbilical cord-derived mesenchymal stem cells (UC-MSCs), and the MSCs in good condition were divided into 1×10 6 Cell culture six-well plate at the density of cells / well, 37°C, 5% CO 2 overnight in the cell culture incubator; according to the method described in the inventor's published literature (Yuan et al., Cytotherapy.2015Jul; 17(7):885-96.doi:10.1016 / j.jcyt.2015.03.603.), the expression human The full-length TRAIL lentivirus was used at a virus concentration of MOI=3, and 8 μg / mL polybrene was used to enhance transfection, and TRAIL genetic engineering was performed on MSCs. After transfection and incubation for 6-24 hours (optimally 10 hours), high Cells expressing full-length TRAIL, that is, MSCflT; replace with fresh DMEM / F12 medium containing 10% FBS (fetal bovine serum), and continue to cult...
Embodiment 2
[0037] Example 2 Preparation and identification of MSC extracellular vesicles (EV-T) expressing TRAIL
[0038] 1. EV-T preparation: thaw the frozen MSCflT cell culture supernatant at 4°C, first centrifuge at low speed (4°C, 10min, 1000g) to remove dead cells and cell debris, and filter through a 0.22μm filter membrane to remove cells larger than 220nm Then use 100kD ultrafiltration centrifugation (4°C, 3000g, 10min) to concentrate the EV-T solution 3 times, and finally precipitate EV by ultracentrifugation (4°C, 120000g, 2h) -T, resuspend the EV-T pellet in PBS solution filtered through a 0.22 μm filter membrane, aliquot and freeze at -80°C for future use.
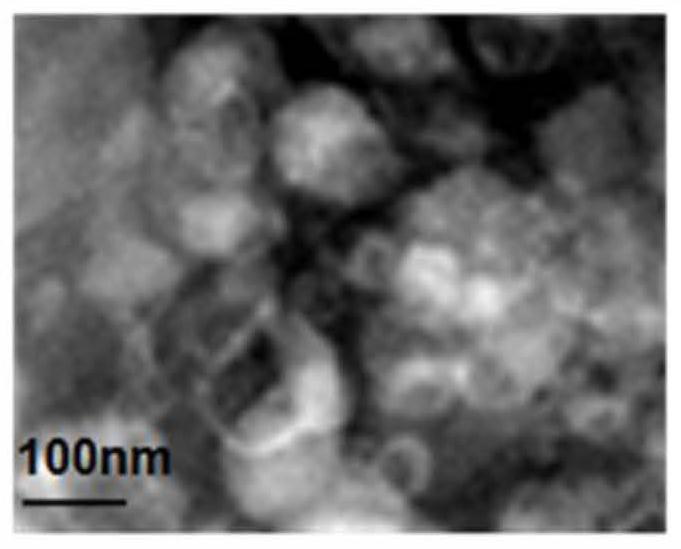
[0039] 2. Observation of EV-T morphology: Observe the morphology and size of the prepared EV-T through a transmission electron microscope. The results are as follows: figure 2 As shown, it was observed that EV-T presents a typical membrane vesicle structure of extracellular vesicles, with a diameter of about 60-100nm.
Embodiment 3
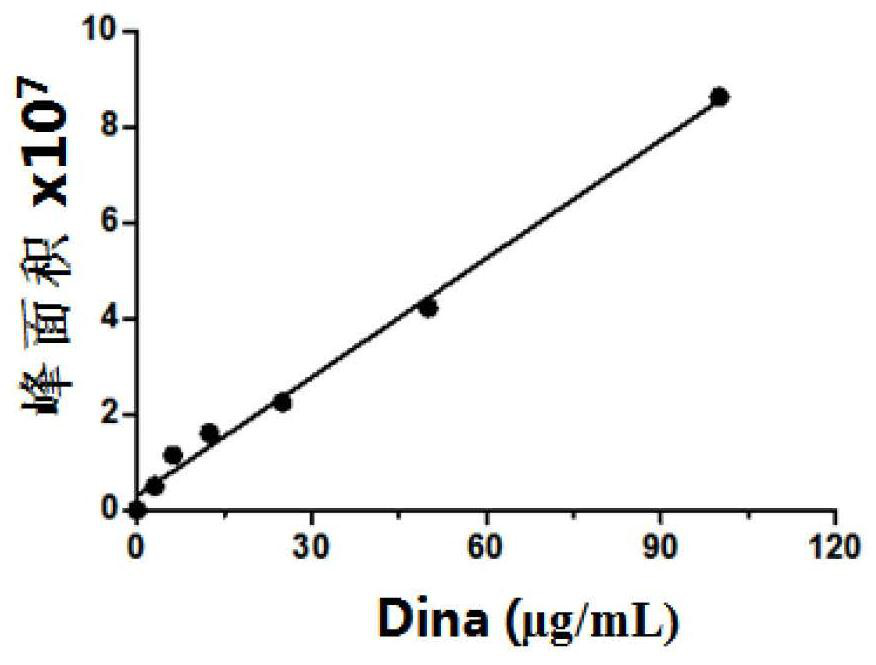
[0040] Embodiment 3 establishes the standard curve of HPLC quantitative determination Dina
[0041] First prepare 100μg / mL, 50μg / mL, 25μg / mL, 12.5μg / mL, 6.25μg / mL and 3.125μg / mL Dina standard samples for establishing the standard curve. The model of the chromatographic column is selected as C18 (4.6*150mm) and the particle size of the filler is 5 μm according to the references, and the mobile phase composition of the chromatographic analysis is determined as: A phase H 2 O (add 0.05% H 3 PO 4 ), phase B HPLC grade acetonitrile (adding 0.05% H 3 PO 4 ), the column temperature was 40°C, and the flow rate was 0.8mL / min. Mobile phase gradient time: 0~6min grade (5%B), 6.1~15min grade (5%~95%B), 15.1~20min grade (95%B), 20.1~25min (5%B). The detection wavelength is 220 / 254nm, and the injection volume is 20μl. HPLC detects that the main peak of its Dina appears at about 11.5min, and the standard curve result of detecting Dina is as follows image 3 As shown, the fitted standa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com