Salbutamol sulfate solution for inhalation and preparation method thereof
A technology of salbutamol sulfate and solution, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of unencapsulated, uneven encapsulation, troublesome operation, etc., and achieve Accurate control of inhalation volume, uniform drug content, and less impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
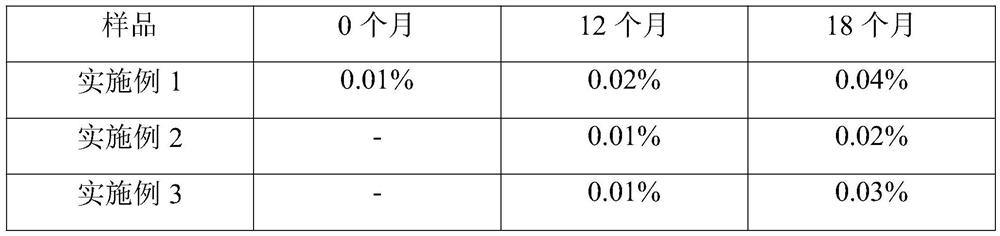
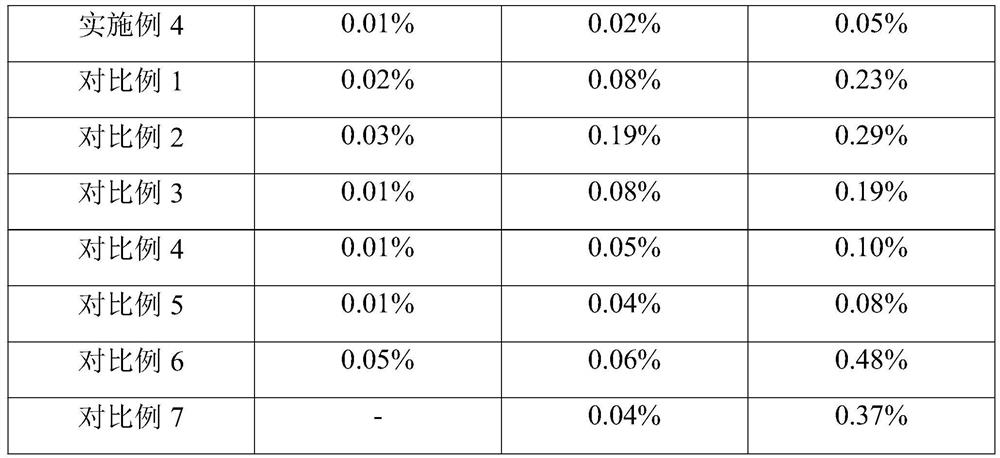
Examples
Embodiment 1
[0031] The composition of the prescription is as follows:
[0032] Salbutamol sulfate 0.5g;
[0033] Lactose 5g;
[0035] Add water for injection to 1000mL.
[0036] Add sodium chloride to water for injection, stir until it is completely dissolved and blow nitrogen gas for 25 minutes; then add lactose, and use CO 2 Lead into equipment and feed carbon dioxide gas until the pH of the solution reaches 5.6 (in the process, adjust the flow rate of carbon dioxide entering the device body 2 by the flow regulating valve 4 to be 15L / min), then add salbutamol sulfate and stir until completely dissolved, and use sulfuric acid to Adjust the pH value to 4.0; finally, filter the prepared solution through a 0.22 μm microporous membrane to sterilize. After the semi-finished product passes the inspection, it is filled with nitrogen and sealed, and sterilized at 121°C for 15 minutes. After passing the quality inspection, the finished product is ready for inhalati...
Embodiment 2
[0038] The composition of the prescription is as follows:
[0039] Salbutamol sulfate 3g;
[0040] Lactose 8g;
[0041] Glucose 8g;
[0042] Add water for injection to 1000mL.
[0043] Add glucose to water for injection, stir until completely dissolved and blow nitrogen for 35 minutes; then add lactose, and use CO 2 Pass into equipment and feed carbon dioxide gas until the pH of solution reaches 5.6 (in this process, regulate the carbon dioxide flow rate that enters in equipment body 2 by flow regulating valve 4 and be 35L / min), then add albuterol sulfate and stir until completely dissolving, use citrate Citric acid-sodium citrate adjusts the pH value to 5.0; finally, the prepared solution is sterilized by filtration through a 0.22 μm microporous membrane. After passing the test, the finished product salbutamol sulfate solution for inhalation is obtained.
Embodiment 3
[0045] The composition of the prescription is as follows:
[0046] Salbutamol sulfate 2.5g;
[0047] Lactose 7g;
[0048] Magnesium chloride 7.3g;
[0049] Add water for injection to 1000mL.
[0050] Add magnesium chloride to water for injection, stir until it is completely dissolved and blow nitrogen gas for 30 minutes; then add lactose, and use CO 2 Lead into the equipment and feed carbon dioxide until the pH of the solution reaches 5.6 (in this process, the flow rate of carbon dioxide entering the device body 2 is adjusted to be 20L / min by the flow regulating valve 4), then add salbutamol sulfate and stir until completely dissolved, and use acetic acid Adjust the pH value to 5.2; finally, filter the prepared solution through a 0.22 μm microporous membrane to sterilize. After the semi-finished product passes the inspection, it is filled with nitrogen and sealed, and sterilized at 121°C for 15 minutes. After passing the quality inspection, the finished product is ready for i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com