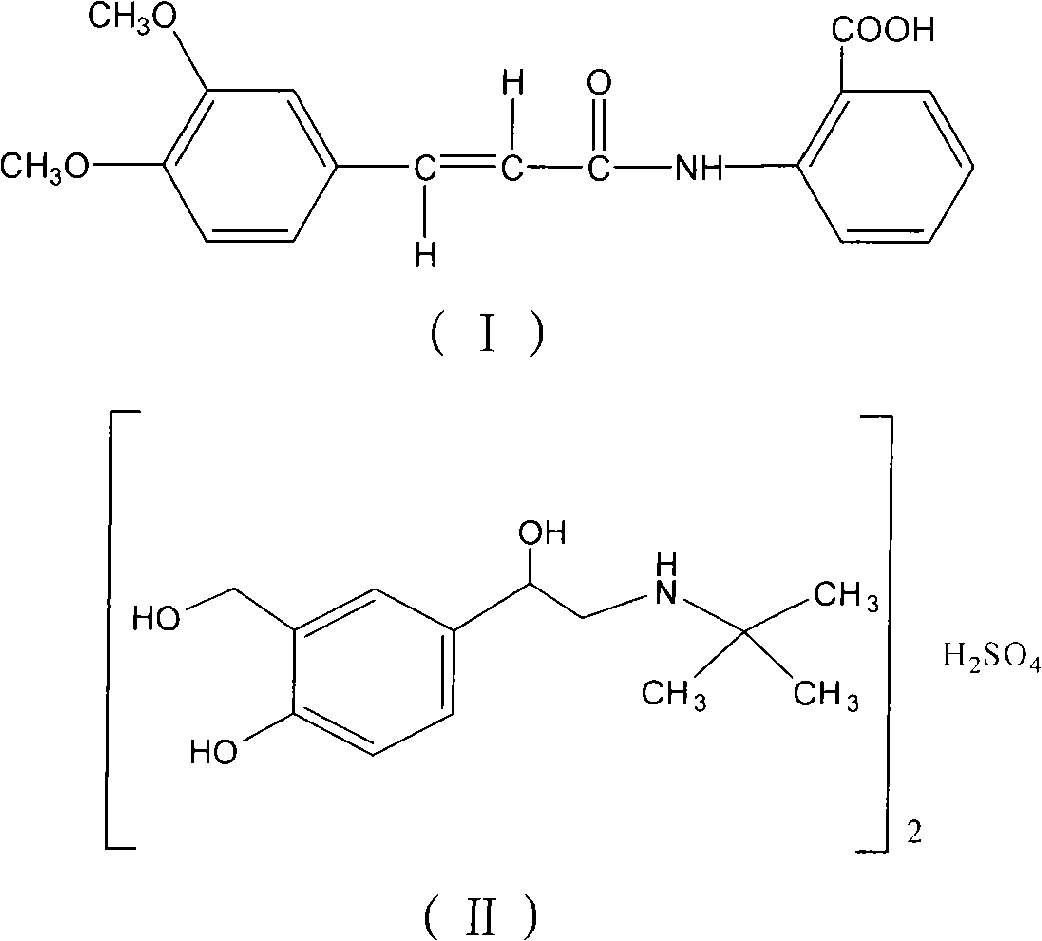
Oral compound pharmaceutic preparation containing tranilast and salbutamol
A pharmaceutical preparation, the technology of salbutamol, which is applied in the field of preparing oral therapeutic agents for asthma, can solve the problems of slow release, difficulty in achieving complete release, and affecting drug efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: compound tranilast dispersible tablet
[0021] 【prescription】
[0022] Tranilast 80.0g
[0023] Salbutamol Sulfate 2.4g
[0024] Starch 81.5g
[0025] Microcrystalline Cellulose 20.0g
[0026] Low-substituted hydroxypropyl cellulose 2.0g
[0027] Sodium carboxymethyl starch 8.0g
[0028] Magnesium Stearate 1.0g
[0030]
[0031] Makes 1000 pieces
[0032] 【Preparation Process】
[0033] All raw and auxiliary materials were crushed through a 100-mesh sieve for later use, and 5% starch slurry was prepared for later use; Tranilast, albuterol sulfate, low-substituted hydroxypropyl cellulose, sodium hydroxymethyl starch, microcrystalline cellulose, and starch were fully mixed and uniform , add 5% starch slurry to moisten, pass through a 18-mesh sieve, dry at 50°C until dry, pass through a 20-mesh sieve for granulation, add magnesium stearate, mix evenly, and press into tablets to...
Embodiment 2
[0040] Embodiment 2: compound tranilast chewable tablet
[0041] 【prescription】
[0042] Tranilast 80.0g
[0043] Salbutamol Sulfate 2.4g
[0044] Mannitol 200.0g
[0045] Microcrystalline Cellulose 80.0g
[0047] Spearmint oil 0.1g
[0048] Magnesium Stearate 5.0g
[0049] 5% polyethylene glycol 6000 ethanol (50%) solution
[0050]
[0051] Made into 1000.0 pieces
[0052] 【Preparation Process】
[0053] Grind the raw materials and auxiliary materials through an 80-mesh sieve for later use; mix tranilast and mannitol evenly in equal increments, and pass through a 60-mesh sieve twice for later use; dissolve salbutamol and sodium saccharin in a small amount of distilled water Then mix it with quantitative 3% hydroxypropyl methylcellulose as a binder. Use the above-mentioned binder to make soft materials, make granules with a 16-mesh sieve, and dry at 60°C to obtain dry granules. The...
Embodiment 3
[0058] Embodiment 3: compound tranilast effervescent tablet
[0059] 【prescription】
[0060] Tranilast 80.0g
[0061]Salbutamol Sulfate 2.4g
[0062] Citric acid 80.0g
[0063] Lactose 44.0g
[0064] Natural Orange Flavor 1.5ml
[0065] Sodium bicarbonate (fine powder) 75.0g
[0066] Magnesium Stearate 3.0g
[0067] 5% polyvinylpyrrolidone K30 appropriate amount
[0068]
[0069] Makes 1000 pieces
[0070] 【Preparation Process】
[0071] The raw and auxiliary materials in the prescription were crushed separately and passed through a 80-mesh sieve for later use; Tranilast, salbutamol sulfate, citric acid, etc. were fully mixed according to the amount of the prescription, and mixed through a 60-mesh sieve twice. Use 5% polyvinylpyrrolidone K30 aqueous solution to make the above mixture into a soft material, use a 18-mesh sieve to wet-process granules, and dry the wet granules at 60° C. to obtain dry granules. After siz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com