Method for synchronously detecting five related substances in compound ipratropium bromide solution for inhalation
A technology of ipratropium bromide and related substances, which is applied in the field of simultaneous detection of five related substances in the compound ipratropium bromide solution for inhalation, can solve the problem of inability to guarantee the measurement sensitivity and specificity, and the inability of related substances and main components Separation, complex components, etc., to avoid changing liquid phase conditions, stable and reliable results, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments and conditions: Waters liquid chromatography system, 2998 detector, chromatographic column: Waters Xbridge C18 (150×4.6mm, 3.5μm); detection wavelength: 210nm; pH adjustment with phosphoric acid in buffer containing 0.006mol / L sodium heptanesulfonate To 2.5 is the mobile phase A, acetonitrile is used as the mobile phase B, the detection wavelength is 210nm, the column temperature is 35°C, and the flow rate is 1.0ml / min.
[0037] In terms of volume ratio, the setting of the gradient elution is:
[0038]
[0039] experiment procedure:
[0040] Among them, ipratropium bromide and its related impurity reference substance were purchased from Zhejiang Youlian Pharmaceutical Chemical Co., Ltd.; salbutamol sulfate and its related impurity reference substance were purchased from Beijing Huawei Ruike Chemical Co., Ltd.; Produced by New Times Pharmaceutical Co., Ltd. (batch number 06418003).
[0041] Take ipratropium bromide, salbutamol sulfate, ipratropium bromid...
Embodiment 2
[0067] Embodiment 2 specificity experiment
[0068] Instruments and conditions: Waters liquid chromatography system, 2998 detector, chromatographic column: Waters Xbridge C18 (150×4.6mm, 3.5μm); detection wavelength: 210nm; pH adjustment with phosphoric acid in buffer containing 0.006mol / L sodium heptanesulfonate To 2.5 is the mobile phase A, acetonitrile is the mobile phase B, the detection wavelength is 210nm, the column temperature is 35°C, and the flow rate is 1.0ml / min.
[0069] In terms of volume ratio, the setting of the gradient elution is:
[0070]
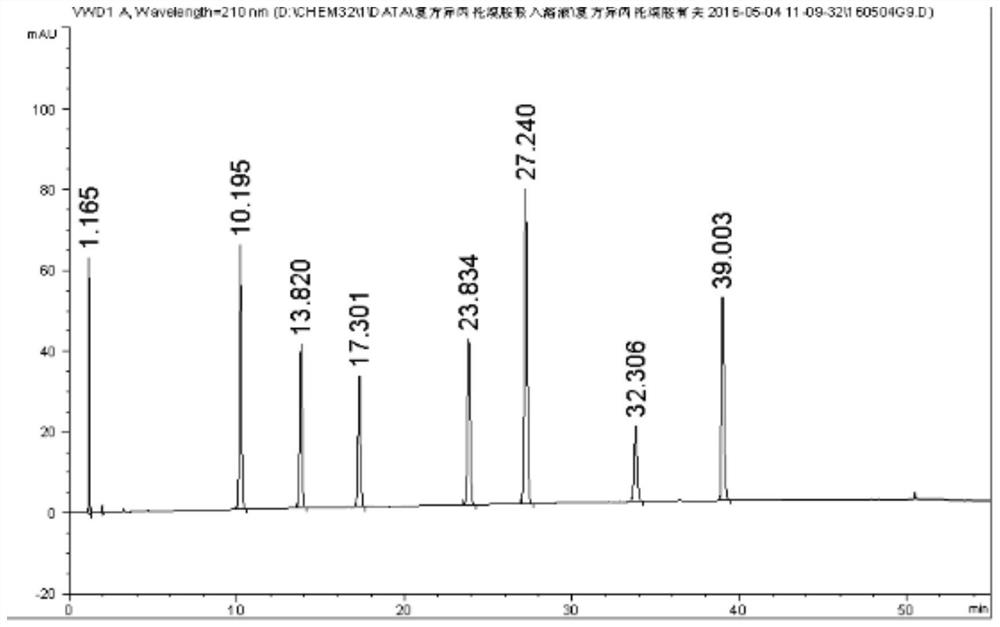
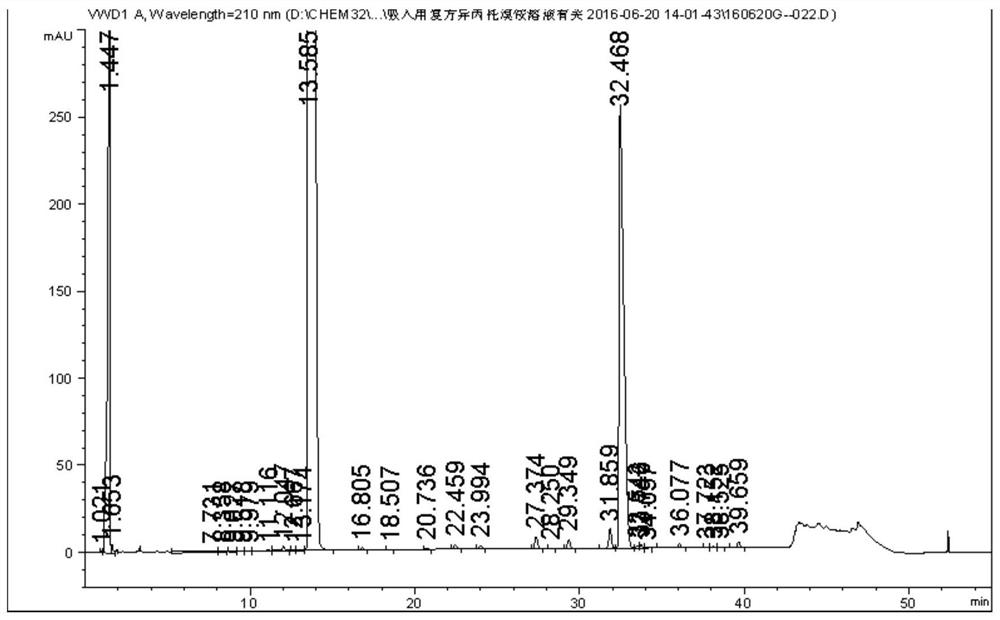
[0071] 1. The compound ipratropium bromide solution blank adjuvant for inhalation (from Shandong New Times Pharmaceutical Co., Ltd.), is determined according to the method of Example 2, and the HPLC spectrum is shown in the appendix Figure 5 .
[0072] Depend on Figure 5 It can be seen that the blank excipients have no interference to the determination of related substances in the samples.
[0073] 2. Determinati...
Embodiment 3
[0075] Embodiment 3 method durability test
[0076] Instruments and conditions: Waters liquid chromatography system, 2998 detector, chromatographic column: Waters Xbridge C18 (150×4.6mm, 3.5μm); detection wavelength: 215nm; pH adjustment with phosphoric acid in buffer containing 0.006mol / L sodium heptanesulfonate To 2.5 is the mobile phase A, methanol is the mobile phase B, the column temperature is 30°C, and the flow rate is 0.9ml / min.
[0077] In terms of volume ratio, the setting of the gradient elution is:
[0078]
[0079] Take ipratropium bromide, albuterol sulfate, ipratropium bromide impurity C, albuterol impurity B, albuterol impurity C, albuterol impurity D, albuterol impurity F and prepare 10 μg each with heptanesulfonate sodium phosphate buffer solution-methanol mixed solution / ml of the mixed solution, accurately measure 25 μl into the liquid chromatograph, and record the chromatogram.
[0080] Under this condition, the retention time of the main peak of ipra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com