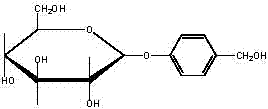
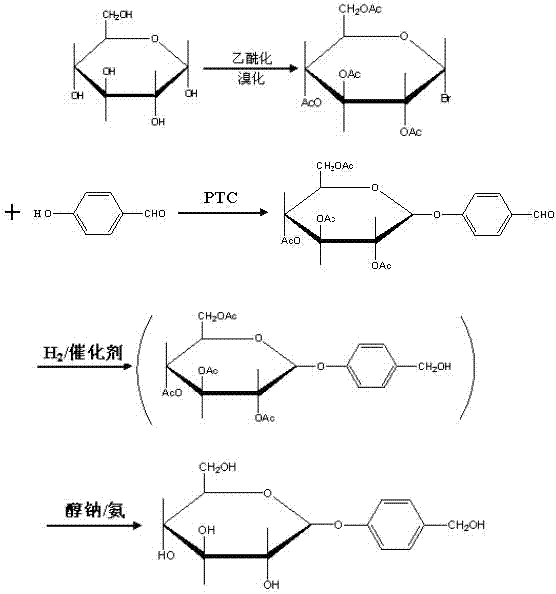
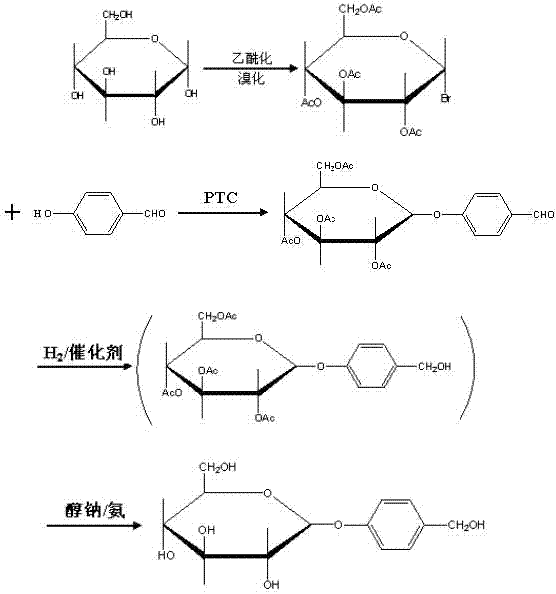
Gastrodin synthesizing method
A synthesis method, the technology of gastrodin, applied in the field of medicine, can solve the problems of high environmental pollution, easy fire, serious human injury, etc., and achieve the effect of high product quality, low environmental pollution and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] When the present invention is specifically prepared, it can be realized by the following steps:
[0024] (1) Control the temperature at 35°C, add catalyst perchloric acid, acetylate anhydrous glucose with acetic anhydride to generate full acetyl sugar, detect the end point of the reaction by TLC, pass hydrogen bromide (gas) after the acetylation is completed, and control At a temperature of 30°C, hemiacetal hydroxybromination of peracetyl sugar produces bromotetraacetylglucose, and the molar ratio of anhydrous glucose: acetic anhydride: perchloric acid: hydrogen bromide = 1: 7.5: 0.04: 0.76;
[0025] (2) Add phase transfer catalysts tetrabutylammonium bromide, carbonate and p-hydroxybenzaldehyde in a two-phase system composed of chloroform and water at a volume ratio of 1:1, control the temperature at 60°C, bromotetraacetylglucose After being dissolved in chloroform, add dropwise, the weight ratio of bromotetraacetylglucose to p-hydroxybenzaldehyde is 1:0.9, TLC monitor...
Embodiment 2
[0030] When the present invention is specifically prepared, it can also be realized by the following steps:
[0031] (1) Control the temperature at 32°C, add catalyst perchloric acid, acetylate anhydrous glucose with acetic anhydride to generate full acetyl sugar, detect the end of the reaction by TLC, pass hydrogen bromide (gas) after the acetylation is completed, and control At a temperature of 28°C, hemiacetal hydroxy bromide of peracetyl sugar is used to synthesize bromotetraacetylglucose, and the molar ratio of anhydrous glucose: acetic anhydride: perchloric acid: hydrogen bromide = 1: 7.5: 0.04: 0.76;
[0032](2) Add the phase transfer catalyst tetrabutylammonium bromide, carbonate and p-hydroxybenzaldehyde in the two-phase system composed of chloroform and water with a volume ratio of 1:1, control the temperature at 55 ° C, bromotetraacetylglucose After dissolving in chloroform, add dropwise, the weight ratio of bromotetraacetylglucose to p-hydroxybenzaldehyde is 1:1, a...
Embodiment 3
[0037] When the present invention is specifically prepared, it can also be realized by the following steps:
[0038] (1) Control the temperature at 31°C, add catalyst perchloric acid, acetylate anhydrous glucose with acetic anhydride to generate full acetyl sugar, detect the end point of the reaction by TLC, pass hydrogen bromide (gas) after the acetylation is completed, and control At a temperature of 27°C, hemiacetal hydroxy bromide of peracetyl sugar is used to synthesize bromotetraacetylglucose, and the molar ratio of molar ratio of anhydrous glucose: acetic anhydride: perchloric acid: hydrogen bromide = 1: 7.5: 0.04: 0.76;
[0039] (2) Add phase transfer catalysts tetrabutylammonium bromide, carbonate and p-hydroxybenzaldehyde in a two-phase system composed of chloroform and water at a volume ratio of 1:1, control the temperature at 50°C, bromotetraacetylglucose After dissolving in chloroform, add dropwise, the weight ratio of bromotetraacetylglucose to p-hydroxybenzaldeh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com