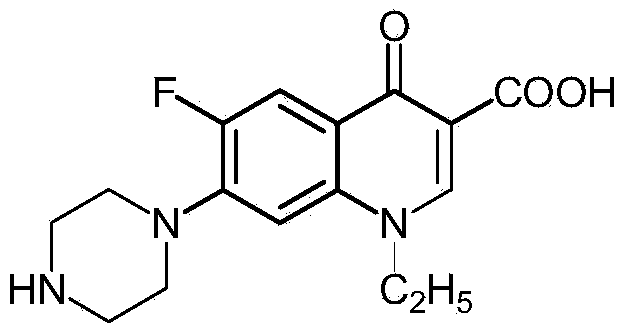
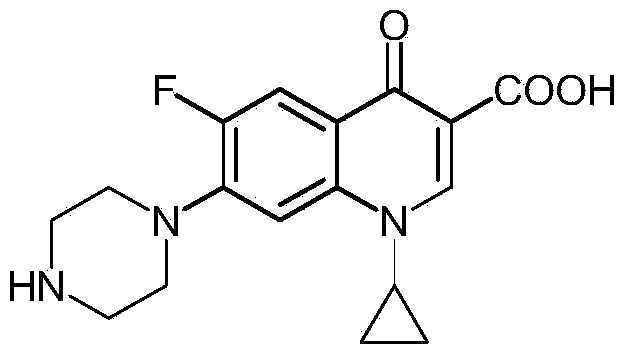
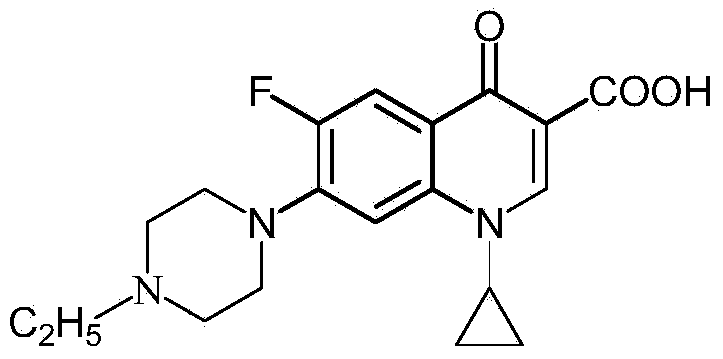
Preparation method of norfloxacin, ciprofloxacin and enrofloxacin
A technology of norfloxacin and ciprofloxacin is applied in the field of drug synthesis, and can solve the problems of high cost, increased consumption of N-methylpiperazine, low efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Step (1) At room temperature, add 40g (0.176mol) of 2,4-dichloro-5-fluorobenzoyl chloride and 36g (0.195mol) of tri-n-butylamine into the reaction flask, and add N,N-dimethylamino under stirring 25.5g (0.178mol) of ethyl acrylate, the reaction is exothermic by itself, the temperature of the reaction is controlled by cooling with cold water to about 70°C, and after the temperature is stable, it is then kept and stirred for 1 hour, and the reaction is completed. Add 100ml of xylene and 50ml of distilled water to the reaction solution, add hydrochloric acid to adjust the pH to 1-2, separate the organic layer, wash with water until neutral, evaporate the remaining water, transfer to another reaction bottle, and add ring Propylamine 11g (0.194mol), and pass into CO 2 gas, control the reaction pressure to 2 atm, and track it by HPLC. After the reaction is complete, the N,N-dimethylamine complex is recovered by distillation to obtain the mother liquor for future use.
[0063]...
Embodiment 2
[0066] Step (1) At room temperature, add 40g (0.176mol) of 2,4-dichloro-5-fluorobenzoyl chloride and 36g (0.195mol) of tri-n-butylamine into the reaction flask, and add N,N-dimethylamino under stirring 25.5g (0.178mol) of ethyl acrylate, the reaction is exothermic by itself, the temperature of the reaction is controlled by cooling with cold water to about 70°C, and after the temperature is stable, it is then kept and stirred for 1 hour, and the reaction is completed. Add 100ml of xylene and 50ml of distilled water to the reaction solution, add hydrochloric acid to adjust the pH to 1-2, separate the organic layer, wash with water until neutral, evaporate the remaining water, transfer to another reaction bottle, and add ring Propylamine 11g (0.194mol), and pass into CO 2 gas, control the reaction pressure to 2 atm, and track it by HPLC. After the reaction is complete, the N,N-dimethylamine complex is recovered by distillation to obtain the mother liquor for future use.
[0067]...
Embodiment 3
[0070] Step (1) At room temperature, add 40g (0.176mol) of 2,4-dichloro-5-fluorobenzoyl chloride and 32.7g (0.176mol) of tri-n-butylamine into the reaction flask, and add N,N-dimethyl 25.2 g (0.176 mol) of ethyl aminoacrylate, the reaction is exothermic by itself, cooled with cold water to control the reaction temperature to about 40°C, after the temperature stabilizes, keep stirring for 1 hour, and the reaction ends. Add 80ml of xylene and 40ml of distilled water to the reaction solution, add hydrochloric acid to adjust the pH to 1-2, separate the organic layer, wash with water until neutral, evaporate the remaining water, transfer to another reaction bottle, and add ring Propylamine 8g (0.141mol), and pass into CO 2 Gas, the reaction pressure is controlled to 1 atm, followed by HPLC, after the reaction is complete, the N,N-dimethylamine complex is recovered by distillation, and the mother liquor is obtained for future use.
[0071] Step (2) Add 80ml of xylene and 14.8g (0.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com