Preparation method of Dapagliflozin eutectic matter
A preparation process and co-crystal technology, which is applied in the direction of drug combinations, carbohydrate active ingredients, metabolic diseases, etc., can solve the problems of cumbersome treatment of pharmaceutical crystal solvent residues, cumbersome synthesis process and post-processing, and complicated reaction steps. Achieve the effect of convenient industrial production, low equipment requirements and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
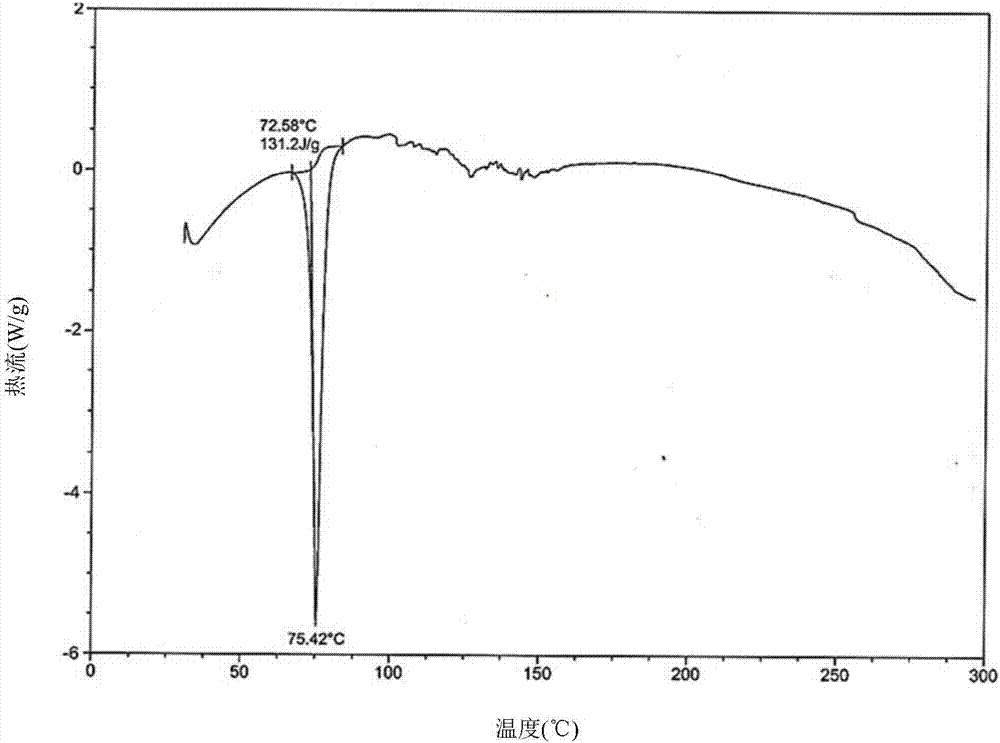
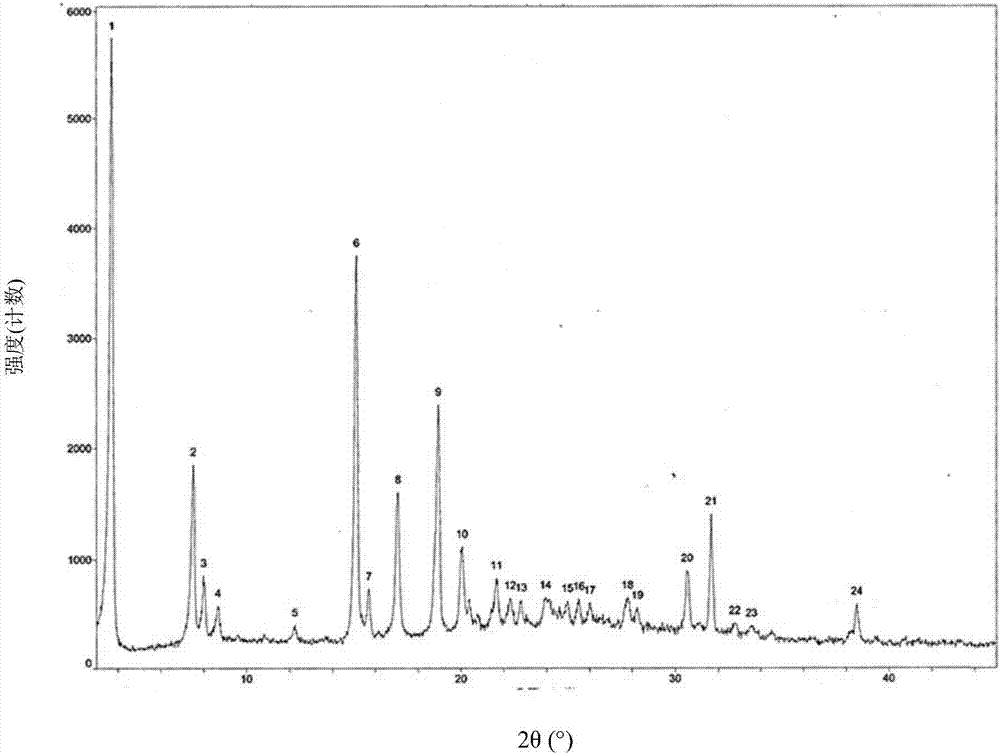
Image
Examples
Embodiment 2
[0059] Example 2, (1S)-2,3,4,6-tetra-O-pivaloyl-1,5-anhydro-1-[3-(4-ethoxyphenylmethyl)-4-chloro Preparation of phenyl]glucitol (compound 3)
[0060] Add n-butyl ether (30mL) to zinc bromide (2.25g) and lithium bromide (0.87g), heat to 50°C and stir for 2h, cool for later use. Under nitrogen protection, add toluene (10mL) and n-butyl ether (10mL) to 4-(2-chloro-5-iodo-benzyl)phenetole (7.45g), cool to -20°C, and slowly add 1.6mol / L n-hexyllithium n-hexane solution (14mL), control the internal temperature not to exceed -10°C, after the dropwise addition, keep it at -20°C for 0.5h, add the spare n-butyl ether solution of zinc bromide and lithium bromide, and The reaction was stirred at 20°C for 3h. Add 2,3,4,6-tetra-O-pivaloyl-α-D-bromoglucopyranose (11.59g) toluene (50mL) solution, heat to 120°C and stir for 4h. After the reaction is detected by TLC, Add 1mol / L dilute hydrochloric acid (40mL) and water (20mL) for extraction, wash the organic phase with water (40mL), wash wi...
Embodiment 3
[0061] Example 3, (1S)-2,3,4,6-tetra-O-pivaloyl-1,5-anhydro-1-[3-(4-ethoxyphenylmethyl)-4-chloro Preparation of phenyl]glucitol (compound 3)
[0062] Add n-butyl ether (40mL) to zinc bromide (3.38g) and lithium bromide (1.3g), heat to 50°C and stir for 2h, cool for later use. Under nitrogen protection, add toluene (20mL) and n-butyl ether (5mL) to 4-(2-chloro-5-iodo-benzyl)phenetole (7.45g), cool to -50°C, and slowly add 2.5mol / L n-butyllithium n-hexane solution (8mL), control the internal temperature not to exceed -30°C, after the dropwise addition, keep it at -50°C for 10 hours, add the above-mentioned spare n-butyl ether solution of zinc bromide and lithium bromide, The reaction was stirred at -20°C for 10 h. Add 2,3,4,6-tetra-O-pivaloyl-α-D-bromoglucopyranose (34.77g) toluene (80mL) solution, heat to 100°C and stir for 24h. After the reaction is detected by TLC, Add 1mol / L dilute hydrochloric acid (60mL) and water (50mL) for extraction, wash the organic phase with wate...
Embodiment 4
[0063] Example 4, (1S)-2,3,4,6-tetra-O-pivaloyl-1,5-anhydro-1-[3-(4-ethoxyphenylmethyl)-4-chloro Preparation of phenyl]glucitol (compound 3)
[0064] Add n-butyl ether (50mL) to zinc iodide (3.19g) and lithium iodide (1.34g), heat to 50°C and stir for 1.5h, then cool for later use. Under nitrogen protection, add toluene (15mL) and n-butyl ether (5mL) into 4-(2-chloro-5-iodo-benzyl)phenetole (7.45g), cool to -60°C, and slowly add 1.6mol / L n-hexyllithium n-hexane solution (13.8mL), control the internal temperature not to exceed -20°C, after the dropwise addition, keep it at -60°C for 5 hours, add the above-mentioned spare n-butyl ether solution of zinc iodide and lithium iodide , The reaction was stirred at 25 °C for 1 h. Add 2,3,4,6-tetra-O-pivaloyl-α-D-bromoglucopyranose (23.2g) toluene (50mL) solution, heat to 140°C and reflux for 0.5h, after the reaction is detected by TLC , add 1mol / L dilute hydrochloric acid (50mL), water (50mL), extract, the organic phase is washed wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com