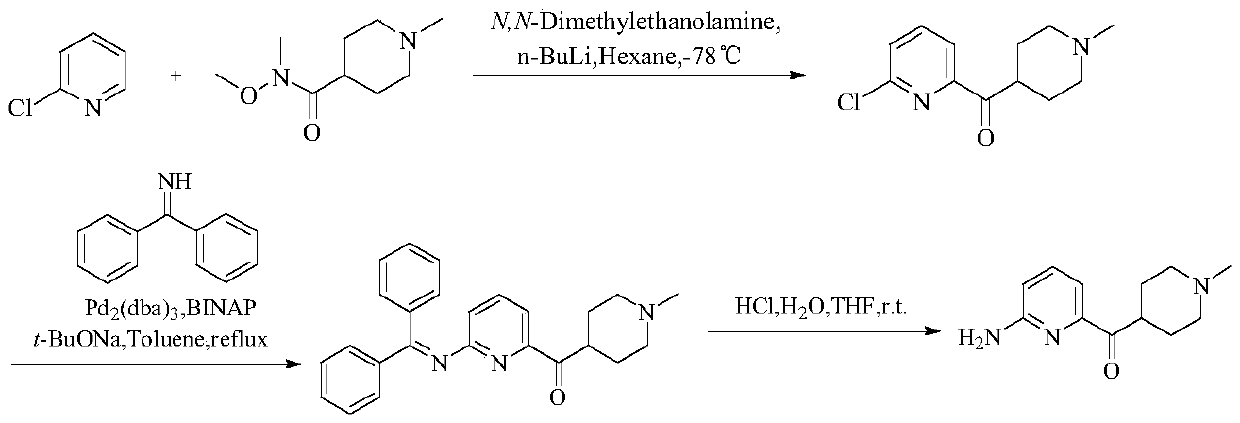
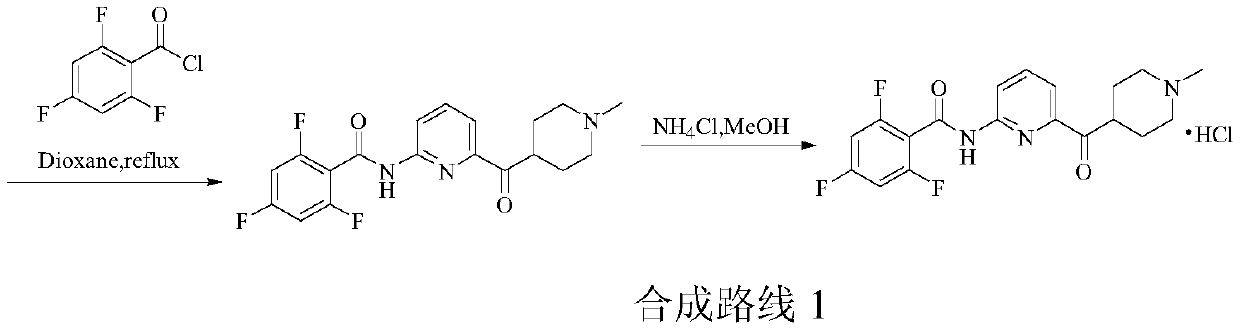
A kind of preparation method of 5-ht1f agonist compound
A compound and solvent technology, applied in the field of medicinal chemistry, can solve problems such as unfavorable industrial production, high raw material prices, and cumbersome processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Preparation of 2-chloro-5,6-dioxo-n-heptanonitrile (Ⅲ1)
[0073] To a 500 ml four-neck flask connected with a stirring, thermometer, and reflux condenser, add 250 g of tetrahydrofuran, 43.0 g (0.5 mole) of 2,3-butanedione (II), 43.5 g (0.5 mole) of 2-chloropropene Nitrile, 0.6 g of lithium hydroxide, 50 to 55 ° C stirring reaction for 4 hours, cooled to 20 to 25 ° C, added 1.0 g of acetic acid, distillation recovery solvent, and then vacuum distillation to collect 90-105 ° C / 1-2mmHg fraction, to obtain 81.2 gram of 2-chloro-5,6-dioxo-n-heptanonitrile (Ⅲ1), the yield was 93.6%, and the gas phase purity was 99.5%.
Embodiment 2
[0074] Example 2: Preparation of 2-bromo-5,6-dioxo-n-heptanonitrile (Ⅲ2)
[0075] To a 500 ml four-neck flask connected with a stirring, thermometer, and reflux condenser, add 250 g of isopropyl ether, 43.0 g (0.5 mol) of 2,3-butanedione (II), 66.0 g (0.5 mol) of 2- Bromoacrylonitrile, 0.6 g of DBU, stirred at 60 to 65°C for 4 hours, recovered the solvent by distillation, and then collected 105-115°C / 1-2mmHg fractions by distillation under reduced pressure to obtain 103.2 g of 2-bromo-5,6-dioxo N-heptanonitrile (Ⅲ2), the yield is 94.7%, and the gas phase purity is 99.2%.
Embodiment 3
[0076] Example 3: Preparation of 2-dimethylmethyleneamino-6-acetylpyridine (V1)
[0077] To a 500 ml four-necked flask connected with stirring, thermometer, reflux condenser and dropping funnel, add 80 g of methanol, 40.0 g (0.4 moles) of 17% ammonia methanol solution, between 50 and 55 ° C, dropwise add 17.4 g (0.1 mole) The solution of 2-chloro-5,6-dioxo-n-heptanonitrile (III1) prepared in Example 1 and 80 g of methanol was added dropwise in about 2 hours, and then stirred at 55 to 60°C for 4 hours. Cool to 20 to 25°C, add the resulting reaction liquid to 200 grams of dichloromethane and 200 grams of ice water, separate layers, extract the water layer with dichloromethane 3 times, 20 grams each time, combine the organic phases, 10.0 grams of anhydrous Sodium sulfate was dried for 4 hours, filtered, and the resulting filtrate was transferred to a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, and 20.0 grams (0.19 moles) of 2,2-dimethoxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com