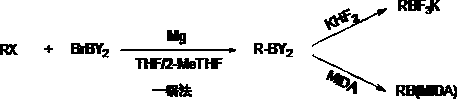A kind of synthetic boron amine compound technology and product application
A compound and boron amine technology, applied in the new process of synthesizing boron amine compounds and product application fields, can solve the problems of heavy metal residues and high cost of boronate esters, improve product yield and purity, reduce operating equipment, and avoid ultra-low temperature reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Compound MeB(N i -Pr 2 ) 2 Synthesis:
[0020] Under nitrogen protection, in a three-necked flask equipped with a dropping device, add magnesium metal (0.11 mol) and 10 ml of tetrahydrofuran, cool down to 0°C, and start to add methyl iodide (0.1 mol) and bis(N,N-diiso) propyl)boron bromide (0.11 mol) was dissolved in 150 mL of tetrahydrofuran. After 15-20 ml was added dropwise, stirring was continued for about 10-20 minutes, and the temperature of the reaction solution rose from 0°C to 6°C, which indicated that the reaction had been initiated. After the addition was completed, the temperature was naturally raised to room temperature and the reaction was continued for 3-5 hours with stirring, and GC confirmed the completion of the reaction. Saturated ammonium chloride was added for quenching, and the pH value of the solution was adjusted to 4-5. After adding ethyl acetate, the layers were separated, the aqueous layer was extracted once more with ethyl acetate, and t...
Embodiment 2
[0022] Compound CH 2 =CHCH 2 B(N i -Pr 2 ) 2 Synthesis:
[0023] Under nitrogen protection, in the there-necked flask equipped with dripping and reflux device, add magnesium metal (0.12 mole), 15 ml of 2-methyltetrahydrofuran and several small grains of iodine, be warming up to 50 ℃, start to drip allyl chloride ( 0.1 mol) and bis(N,N-diisopropyl)boron bromide (0.1 mol) were dissolved in 110 mL of 2-methyltetrahydrofuran solution. After the initial dropwise addition of 15-20 ml, the stirring was continued for about 10-20 minutes, and the purple color in the reaction solution disappeared, indicating that the reaction had been initiated. After the addition was completed, the temperature was maintained and the stirring reaction continued for 3-5 hours, and GC confirmed the completion of the reaction. Saturated ammonium chloride was added for quenching, and the pH value of the solution was adjusted to 4-5. The layers were separated, the aqueous layer was extracted once more...
Embodiment 3
[0025] compound Synthesis:
[0026] Under nitrogen protection, in a three-necked flask equipped with a dripping and reflux device, add metallic magnesium (0.11 mol), 10 milliliters of tetrahydrofuran and a few small grains of iodine, be warming up to 50 ° C, and begin to drip cyclopropyl bromide (0.1 mol) and Bis(tetrahydropyrrole)boron bromide (0.1 mol) was dissolved in 130 mL of tetrahydrofuran solution. After the initial dropwise addition of 15-20 ml, continue stirring for about 5-10 minutes, and the purple color in the reaction solution disappears, indicating that the reaction has been initiated. After the addition was completed, the temperature was raised to reflux and the stirring was continued for 3-5 hours. GC confirmed that the reaction was completed. After cooling, add saturated ammonium chloride to quench, and adjust the pH value of the solution to 4-5. After adding ethyl acetate, the layers were separated, the aqueous layer was extracted once more with ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com