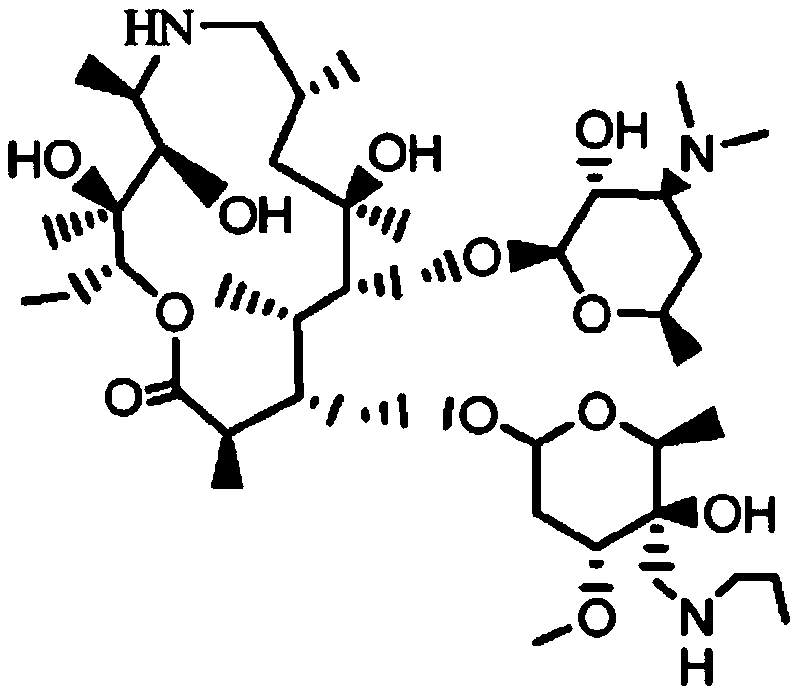
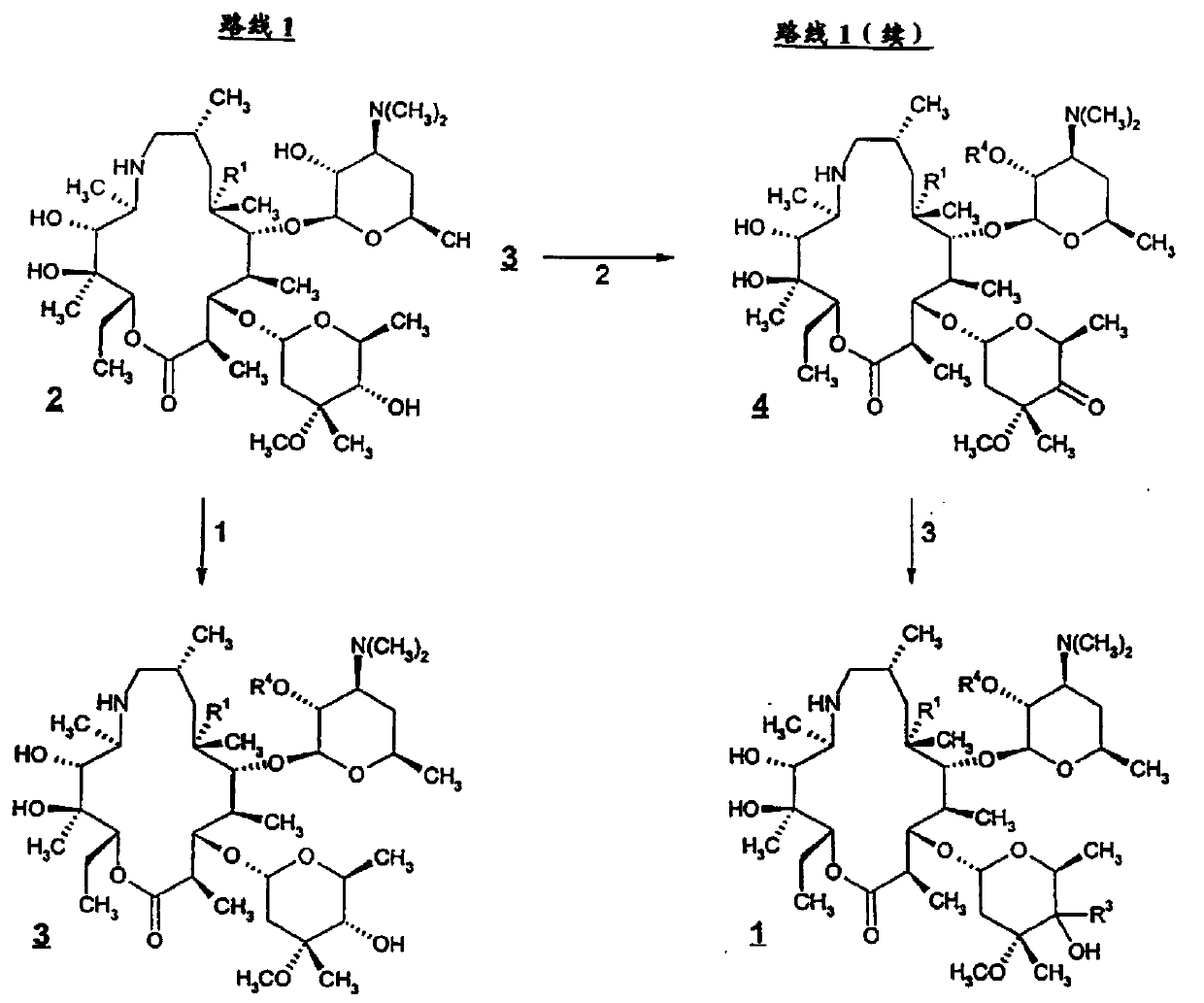
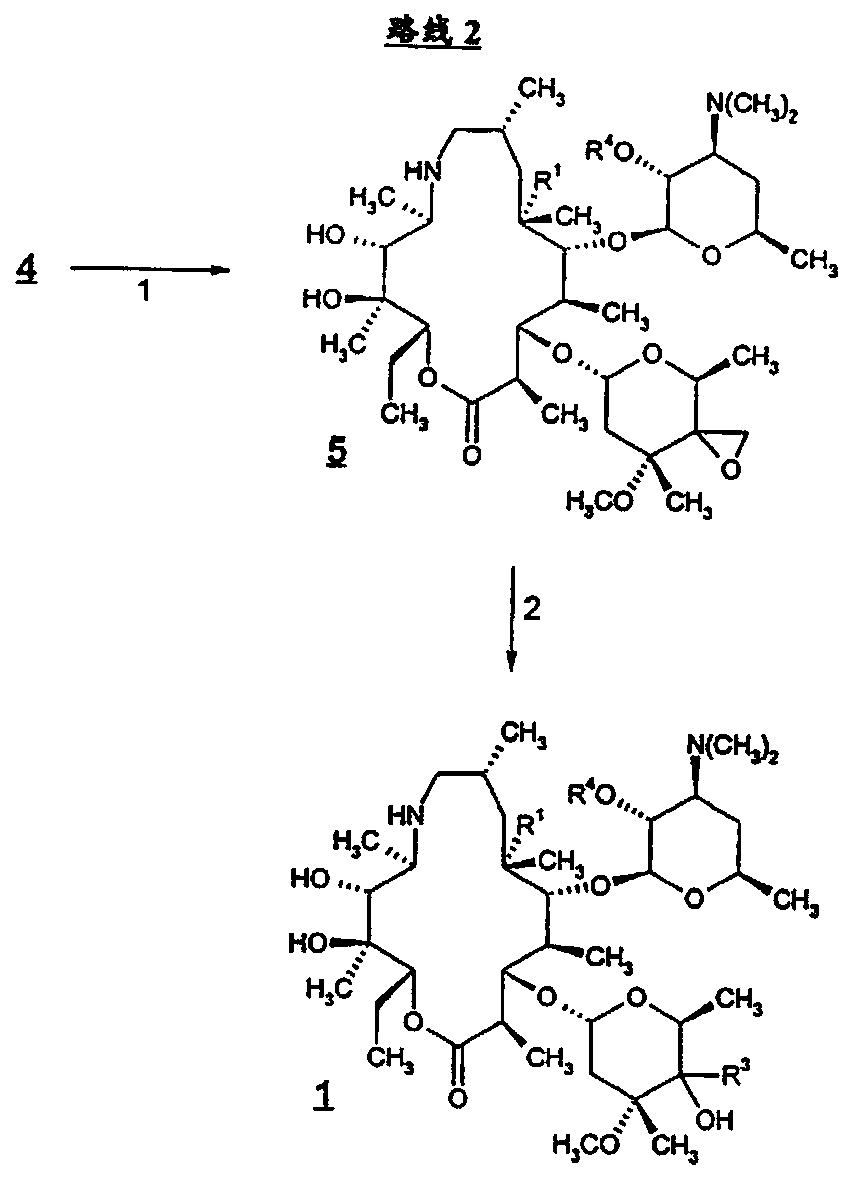
Tyramectin oxalate
A telamectin and oxalate technology, applied in organic chemistry, chemical instruments and methods, sugar derivatives, etc., can solve the problems of safety and cost in industrialization, many reaction by-products, low conversion rate, etc., to avoid Ultra-low temperature reaction, good purity, and the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The content of the invention will be further explained and illustrated below in conjunction with specific examples, but they do not constitute a restriction or limitation to the scope of the present invention. The following reagents and preparation materials are commercially available unless otherwise specified. Example 1 (comparative example, refer to patent 200510082063.7)
[0048] Put 29Kg of TA04 and 160Kg of dichloromethane into the 500L reaction kettle, stir for 20-30min, put 5Kg of anhydrous sodium sulfate into it, stir and dry for 1-2h, filter the whole mixture with suction, and concentrate to basically no fractions. Transfer the concentrated system to a 1000L reaction kettle, add 660Kg of dichloromethane simultaneously, turn on stirring, and simultaneously cool the system to -5~5°C under nitrogen protection, add 16Kg / 33Kg of benzyl chloroformate and dichloromethane dropwise to the system. Chloromethane solution. A total of 1.5h was added dropwise, and the dropwi...
Embodiment 2
[0060] Embodiment 2 (process of the present invention)
[0061] Put 40kg of TA04 and 100kg of toluene into the 1000L reaction kettle, stir for 20-30min, and concentrate at 55°C until there is no fraction. The temperature was lowered to about 25°C under nitrogen protection, 900Kg of dichloromethane was pumped in under vacuum, stirring was started, the system was cooled to -5~5°C under nitrogen protection, and a solution of 22kg of benzyl chloroformate and 66Kg of dichloromethane was added dropwise to the system. . A total of about 1.5h was added dropwise, and the dropwise addition was completed. Incubate at 0~5℃ for 2~3h, the conversion of raw materials is completed. (Conversion of raw materials is monitored by HPLC) The temperature is controlled to T≤35°C, the reaction mixture is concentrated to dryness and becomes oily, and stored at a low temperature of 0-10°C.
[0062] Add above-mentioned TA05 to 500L reactor, 260kg DMSO, start stirring, after dissolving, add 18Kg IBX to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com