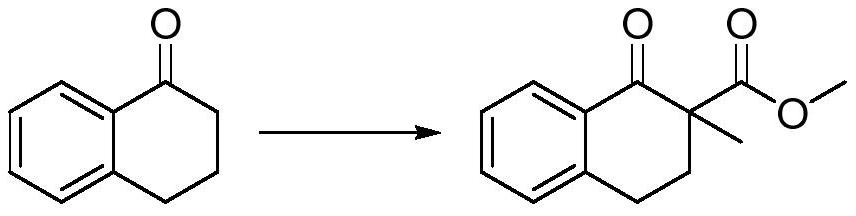
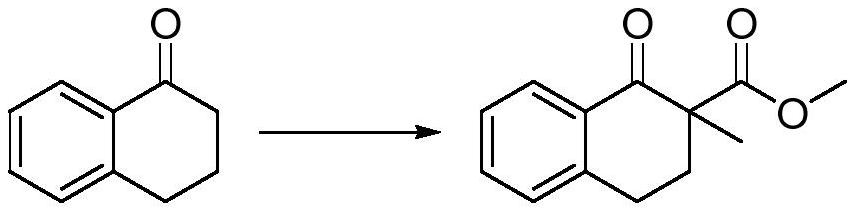
Preparation method of 2-methyl-1-tetralone
A technology of tetralone and methyl, which is applied in the field of synthesis of tetralone derivatives, can solve the problems of low yield of 2-methyl-1-tetralone, cumbersome post-processing steps, column chromatography, etc. , to achieve the effect of short preparation route, avoiding ultra-low temperature reaction, and simple purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The embodiment of the present application provides a preparation method of 2-methyl-1-tetralone, the preparation method comprising:
[0023] Add the dipolar aprotic solvent and sodium hydride into the reaction flask, add 1-tetralone dropwise at 15-35°C, add dimethyl carbonate dropwise after stirring, and control the internal temperature at 45-55°C ℃.
[0024] Cool down to 0-30°C, add methylating reagent dropwise, and place at 0-15°C for 6-18h reaction. The methylating reagent is methyl trifluoroacetate, methyl p-benzenesulfonate or methyl bromide.
[0025] Add acidic aqueous solution dropwise, add water and stir to separate layers, extract with extractant, combine organic phases, and precipitate to obtain a crude product, which is recrystallized to obtain 1-tetralone-2-methyl-2-carboxylic acid methyl ester .
[0026] Add hydrobromic acid to the 1-tetralone-2-methyl-2-carboxylic acid methyl ester, add water, add toluene, add acetic acid, heat, keep the temperature at 9...
Embodiment 1
[0037] Synthesis of 1-tetralone-2-methyl-2-carboxylic acid methyl ester
[0038]
[0039]In a 1000mL four-neck flask, add 320g tetrahydrofuran, add 19.63g (0.5mol, 2.1e.q.) 60% sodium hydride, and add 35g (0.24mol, 1.0e.q.) 1-tetralone dropwise at 25°C After 1 hour, start to add 26g (0.289mol, 1.2e.q.) of dimethyl carbonate dropwise, control the internal temperature between 45-55°C, and complete the addition.
[0040] Cool down to 15°C, and add 68g (0.53mol, 2.2e.q.) of methyl trifluoroacetate dropwise at 15-20°C, and keep the temperature at 25°C for 10h after the dropwise addition.
[0041] Add dropwise 65 g of saturated ammonium chloride aqueous solution to quench below 20°C. Add 200mL of water to stir and separate the layers, separate the liquid, extract the water phase with 150mL of toluene twice, combine the organic phase, force out the water in the organic phase with 150mL of saturated saline, spin the organic phase, crystallize the crude product with 500mL of petrol...
Embodiment 2
[0046] Synthesis of 1-tetralone-2-methyl-2-carboxylic acid methyl ester
[0047]
[0048] In a 2000mL reaction flask, add 600g tetrahydrofuran, add 45g (1.125mol, 2.05e.q.) 60% sodium hydrogen, drop 80g (0.55mol, 1.0e.q.) 1-tetralone at 25°C, and after 1h, start Add 90 g (1.0 mol, 1.8 e.q.) of dimethyl carbonate dropwise, control the internal temperature between 45-55° C., and complete the addition.
[0049] Cool down to 15°C, add 204.8g (1.1mol, 2.0e.q.) methyl p-toluenesulfonate dropwise between 15-20°C, and keep warm at 25°C overnight after the dropwise addition.
[0050] 130 g of saturated ammonium chloride aqueous solution was added dropwise to quench at 20°C or lower. Add 500mL of water and stir to separate the layers, separate the liquids, extract the aqueous phase twice with 350mL toluene, combine the organic phases, add 70g of triethylenediamine to remove excess methyl p-toluenesulfonate, wash the organic phase with 350mL of saturated brine, spin The organic phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com