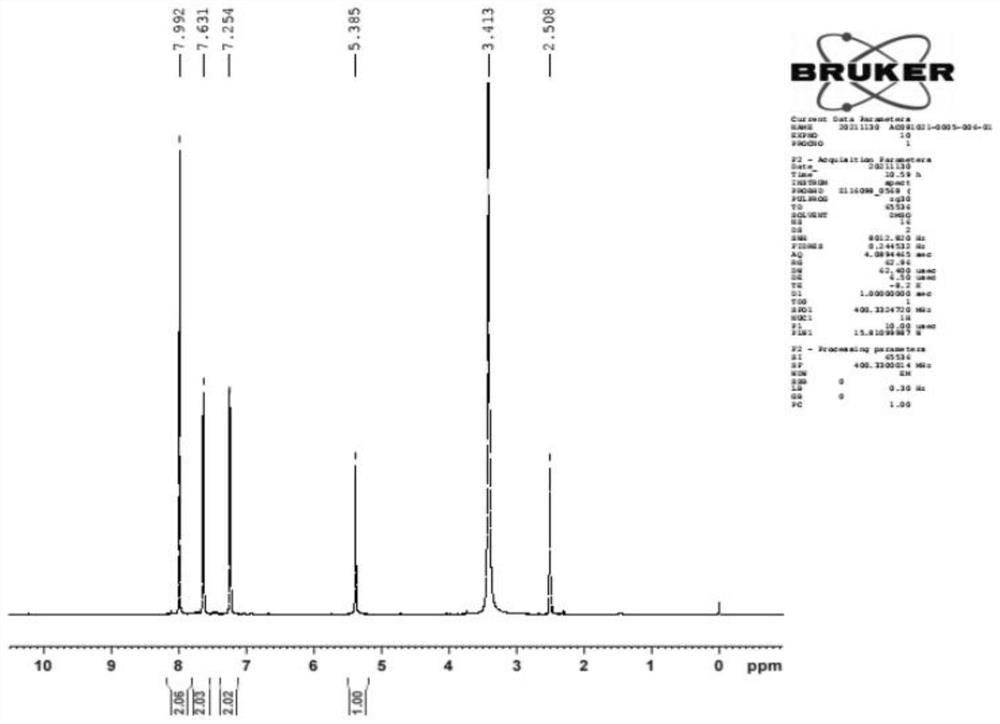
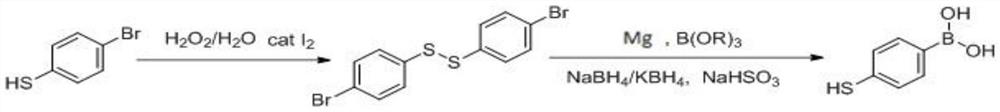
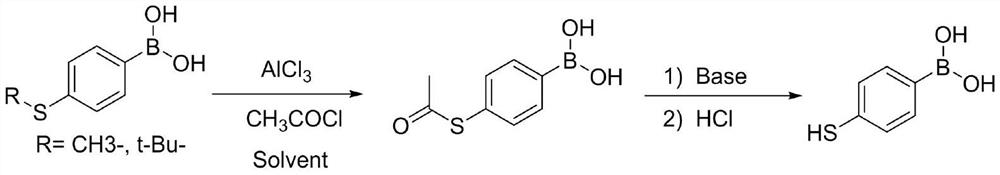
Process method for synthesizing 4-mercaptophenylboronic acid
A technology of mercaptophenylboronic acid and a process method, which is applied in the field of synthesizing 4-mercaptophenylboronic acid, can solve problems such as poor atom economy, low total yield, and poor safety, and achieve simplified operation, increased reaction yield, and shortened reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step A: Add 83.9g (1eq) of p-bromothiophenol, 0.8g (0.007eq) of iodine and 420g of isopropanol into the reaction flask, control the temperature at 50±5°C, and add 27.7g (0.55eq) of 30% hydrogen peroxide dropwise , the dropwise addition was completed, and the reaction was stirred at 50° C. for 1 hour, and a sample was taken by HPLC to confirm the completion of the reaction.
[0027] Post-processing: add 0.5g sodium bisulfite to quench the reaction, after starch potassium iodide test paper detects that there is no peroxide, distill isopropanol under reduced pressure, extract the water layer twice with 150g dichloromethane, combine the organic layer, and add the organic layer Dry over anhydrous sodium sulfate, filter anhydrous sodium sulfate, and evaporate the filtrate to dryness under reduced pressure to obtain 84.3 g of solid product 4,4'-dibromodiphenyl disulfide, with an HPLC purity of 99.2%, and a quantitative yield.
[0028] Step B: Under the protection of nitrogen, ...
Embodiment 2
[0031] Step A: Add 83.9g (1eq) of p-bromothiophenol, 1.1g (0.01eq) of iodine and 420g of ethanol into the reaction flask, control the temperature at 50±5°C, add 33.2g (0.55eq) of 25% hydrogen peroxide dropwise, drop After the addition was complete, the reaction was stirred at 55° C. for 1 hour, and a sample was taken by HPLC to confirm the completion of the reaction.
[0032] Post-treatment: add 1g of sodium bisulfite to quench the reaction, after starch potassium iodide test paper detects that there is no peroxide, distill ethanol under reduced pressure, extract the water layer twice with 150g of ethyl acetate, combine the organic layers, and add anhydrous sulfuric acid to the organic layer Dry over sodium, filter anhydrous sodium sulfate, and evaporate the filtrate to dryness under reduced pressure to obtain 83.5 g of solid product 4,4'-dibromodiphenyl disulfide, with a HPLC purity of 99.4% and a quantitative yield.
[0033] Step B: under the protection of nitrogen, 12.8g (2...
Embodiment 3
[0036] Step A: Add 83.9g (1eq) of p-bromothiophenol, 0.8g (0.007eq) of iodine and 420g of isopropanol into the reaction flask, control the temperature at 50±5°C, and add 25.7g (0.51eq) of 30% hydrogen peroxide dropwise , the dropwise addition was completed, and the reaction was stirred at 55° C. for 1 hour, and a sample was taken by HPLC to confirm the completion of the reaction.
[0037] Post-treatment: add 0.5g sodium bisulfite to quench the reaction, after starch potassium iodide test paper detects that there is no peroxide, distill isopropanol under reduced pressure, extract the water layer twice with 150g toluene, combine the organic layer, add anhydrous Dry over sodium sulfate, filter anhydrous sodium sulfate, and evaporate the filtrate to dryness under reduced pressure to obtain 82.6 g of solid product 4,4'-dibromodiphenyl disulfide, with an HPLC purity of 99.5% and a yield of 99%.
[0038]Step B: Under the protection of nitrogen, 11.6g (2.2eq) of magnesium metal was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com