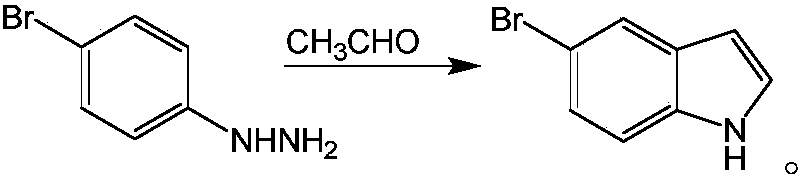
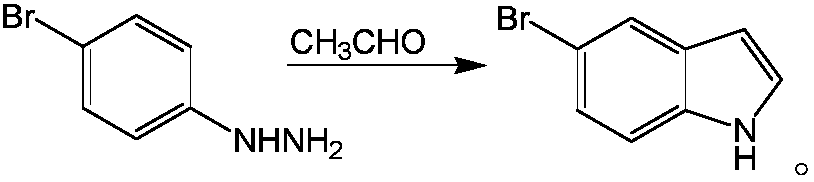
Method for synthesizing 5-bromo-7-azaindole
A kind of technology of azaindole and synthesis method, which is applied in the field of synthesis of 5-bromo-7-azaindole, can solve the problems of a large amount of bromine reaction process, long preparation route, difficult control, etc., and is suitable for large-scale industrial production , simple operation, less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A kind of synthetic method of 5-bromo-7-azaindole, comprises the following steps:
[0021] Dissolve 2-hydrazino-5-bromopyridine in the solvent, add concentrated sulfuric acid, stir and mix evenly, heat to a temperature of 70°C, add catalyst, add acetaldehyde under stirring condition, reflux for 3 hours, the reaction is completed, and lower to room temperature Afterwards, it is filtered, and the filtrate is distilled under reduced pressure and recrystallized to obtain 5-bromo-7-azaindole;
[0022] The catalyst is a mixture of phosphorus oxychloride, copper acetate and niobium pentachloride
[0023]
[0024] Catalyst is the mixture that the molar ratio of phosphorus oxychloride, copper acetate and niobium pentachloride is 4:3:1; Solvent is DMF or DMSO; The usage molar volume ratio of 2-hydrazino-5-bromopyridine and solvent is 2mol / L; the mass ratio of 2-hydrazino-5-bromopyridine to catalyst is 19:15; the molar ratio of 2-hydrazino-5-bromopyridine to acetaldehyde is 1:...
Embodiment 2
[0027] A kind of synthetic method of 5-bromo-7-azaindole, comprises the following steps:
[0028] Dissolve 2-hydrazino-5-bromopyridine in the solvent, add concentrated sulfuric acid, stir and mix evenly, heat to a temperature of 90°C, add catalyst, add acetaldehyde under stirring condition, reflux for 4 hours, the reaction is completed, and lower to room temperature Afterwards, it is filtered, and the filtrate is distilled under reduced pressure and recrystallized to obtain 5-bromo-7-azaindole;
[0029] The catalyst is a mixture of phosphorus oxychloride, copper acetate and niobium pentachloride
[0030]
[0031] Catalyst is the mixture that the molar ratio of phosphorus oxychloride, copper acetate and niobium pentachloride is 4:3:1; Solvent is DMF or DMSO; The usage molar volume ratio of 2-hydrazino-5-bromopyridine and solvent is 3mol / L; the mass ratio of 2-hydrazino-5-bromopyridine to catalyst is 19:18; the molar ratio of 2-hydrazino-5-bromopyridine to acetaldehyde is 1:...
Embodiment 3
[0034] A kind of synthetic method of 5-bromo-7-azaindole, comprises the following steps:
[0035] Dissolve 2-hydrazino-5-bromopyridine in a solvent, add concentrated sulfuric acid, stir and mix well, heat to a temperature of 70°C, add catalyst, add acetaldehyde under stirring condition, reflux for 4 hours, the reaction is completed, and lower to room temperature Afterwards, it is filtered, and the filtrate is distilled under reduced pressure and recrystallized to obtain 5-bromo-7-azaindole;
[0036] The catalyst is a mixture of phosphorus oxychloride, copper acetate and niobium pentachloride
[0037]
[0038] Catalyst is the mixture that the molar ratio of phosphorus oxychloride, copper acetate and niobium pentachloride is 4:3:1; Solvent is DMF or DMSO; The usage molar volume ratio of 2-hydrazino-5-bromopyridine and solvent is 2mol / L; the mass ratio of 2-hydrazino-5-bromopyridine to catalyst is 19:18; the molar ratio of 2-hydrazino-5-bromopyridine to acetaldehyde is 1:1.2;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com