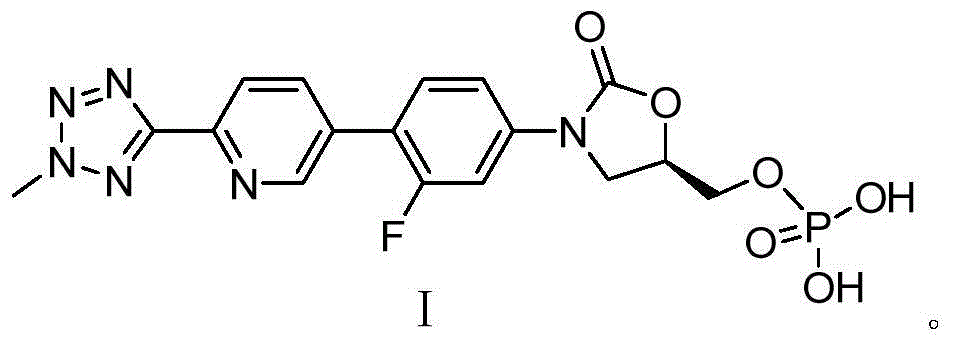
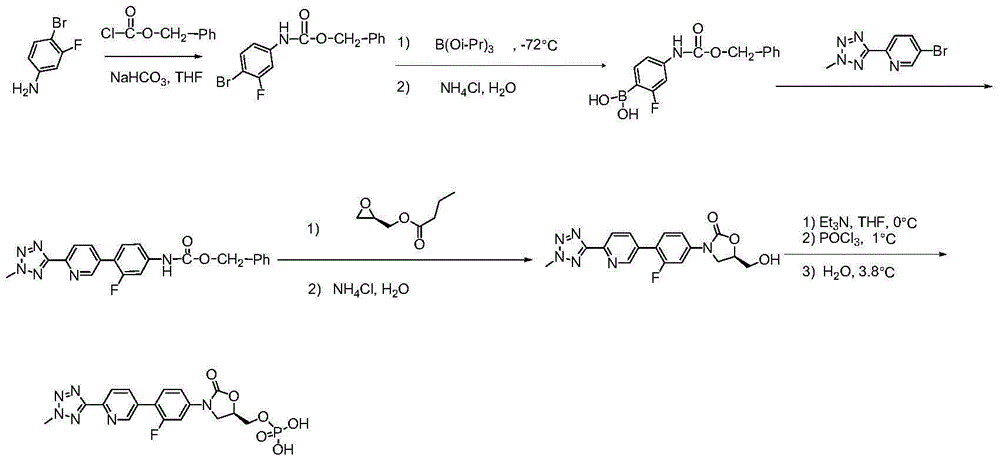
Preparation method for tedizolid phosphate
An independent, cesium carbonate technology, applied in the field of medicine and chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
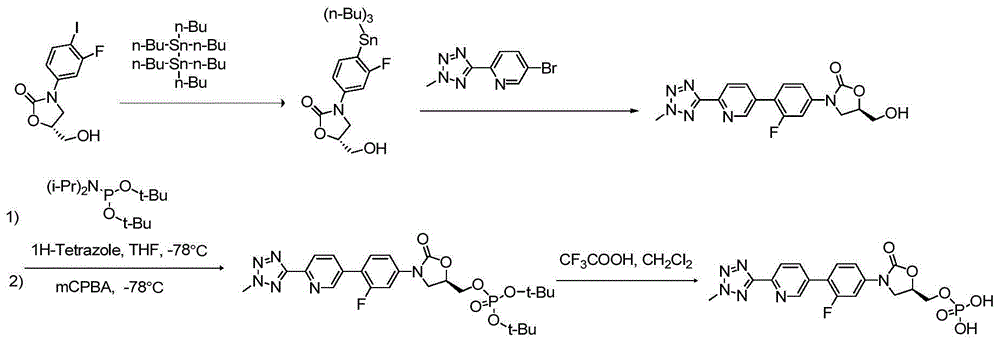
[0070] Example 1(R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)3-fluorophenyl)-5-hydroxymethyloxazolidine-2 - Preparation of ketones (compounds of formula V)
[0071] Add DMSO (100ml), (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (10g, 34.5mmol), biboronic acid to a 250ml reaction flask. Natrol ester (17.52 g, 69 mmol), [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (1.41 g, 1.73 mmol) and potassium acetate (13.5 g) , 138 mmol), under nitrogen protection, the temperature was raised to 80 °C, and the reaction was carried out for 14 hours. The heating was stopped, cooled to room temperature, extracted three times with water / ethyl acetate, and the organic layers were combined, washed three times with saturated brine, dried over anhydrous sodium sulfate, and concentrated by suction filtration.
[0072] The concentrated product of the above step was added to a 250ml reaction flask, 1,4-dioxane (100ml), 5-bromo-2-(2-methyl-2H-te...
Embodiment 2
[0076] Example 2(R)-[3-(4-(2-(2-Methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-2-oxo-5-oxazolidine Preparation of bis(benzyl ester) methyl]methyl phosphate (compound of formula VII)
[0077] Dichloromethane (100ml), 1H-tetrazolium (5.97g, 85.2mmol) and formula V compound (10.5g, 28.4mmol) were added to the 500ml there-necked flask, and diisopropylamino phosphorous acid was added dropwise under temperature control at 30°C Dibenzyl ester (19.6 g, 56.8 mmol) was kept at 25-30 °C for 30 min. The temperature was lowered to 0-10°C, 85% m-chloroperoxybenzoic acid (8.08 g, 39.8 mmol) was added, and the reaction was carried out at 5-10°C for 30 min.
[0078] The reaction solution was successively washed with saturated NaHCO 3 Washed twice, washed once with saturated NaCl, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain 14.89 g of the title compound with a yield of 83.1% and a purity of 99.62% detected by HPLC (area norma...
Embodiment 3
[0082] The preparation of embodiment 3 Tedizolid Phosphate (formula I compound)
[0083] Methanol (1000ml), compound of formula VII (12.8g) and 10% Pd / C (50% water, 1.28g) were added to the 2L reaction flask, hydrogenation reaction was carried out at 50°C under normal pressure for 12h, filtered and evaporated to dryness to obtain 8.78g of the title. The yield of the compound was 96.0%, and the purity detected by HPLC was 99.66% (area normalization method).
[0084] 1 HNMR (500MHz, DMSO-d6): δ8.9367(s, 1H), 8.2052(m, 2H), 7.72(m, 2H), 7.5162(m, 1H), 4.99(m, 1H), 4.4961(s, 3H), 4.2546 (t, 1H), 4.1714 (m, 1H), 4.1035 (m, 1H), 3.9521 (m, 1H).
[0085] 13 CNMR (125MHz, DMSO-d6): δ163.854, 159.2905, 153.925, 149.382, 145.069, 140.286, 137.083, 131.558, 130.87, 122.044, 118.764, 114.079, 105.509, 45.493, 65.559, 45.497
[0086] ESI-MSm / z[M+H] + :451.0927.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com