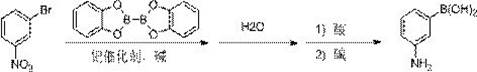
The synthetic method of 3-aminophenylboronic acid
A technology of aminophenylboronic acid and nitrobromobenzene, which is applied in the field of organic boric acid synthesis of pharmaceutical intermediates, can solve the problems of ineffective removal of color, poor reaction reproducibility, and insufficient yield in literature, so as to avoid ultra-low temperature reaction and synthesis Efficiency improvement and overall simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under the protection of nitrogen, 3-nitrobromobenzene (20.2 g, 0.1mol), catechol biborate (52.3g, 0.22mol) and anhydrous potassium acetate (29.4g, 0.3mol) were successively added into the reaction flask , then add 550mL of dioxane, stir evenly, then add PdCl2dppf (0.73g, 1mmol), then slowly raise the temperature to 90°C for reaction under stirring, and stir for 2 hours. At this time, the system is black, and the reaction is detected by TLC. After cooling down to room temperature, 120 mL of water was slowly added dropwise to quench, and after the dropwise addition was completed, the reaction was continued to stir for 4-5 hours. Cool the reaction solution to 0°C, add 10% aqueous hydrochloric acid dropwise to adjust pH=1, add 80 mL of dichloromethane to extract twice, add 20% sodium hydroxide to the aqueous layer to adjust pH=5-6, and extract three times with 120 mL ethyl acetate, Add activated carbon and silica gel to decolorize under reflux, filter and distill ethyl acet...
Embodiment 2
[0025] Under the protection of nitrogen, 3-nitrobromobenzene (20.2 g, 0.1mol), catechol biborate (52.3g, 0.22mol) and anhydrous potassium acetate (29.4g, 0.3mol) were successively added into the reaction flask , Then add 720mL of toluene, stir evenly, then add PdCl2dppf (0.73g, 1mmol), slowly heat up to 110°C under stirring to react, stir for 8-10 hours, the system is black at this time, TLC detects that the reaction is over. After cooling down to room temperature, 110 mL of water was slowly added dropwise to quench, and after the dropwise addition was completed, the reaction was continued to stir for 4-5 hours. Cool the reaction solution to 0°C, add 10% aqueous hydrochloric acid dropwise to adjust the pH=1, discard the organic layer after layering, add 30% potassium hydroxide to the aqueous layer to adjust the pH=5-6, extract three times with 120 mL of ethyl acetate, add Activated carbon and silica gel were refluxed for decolorization. After filtration, ethyl acetate was dist...
Embodiment 3
[0027] Under the protection of nitrogen, 3-nitrobromobenzene (20.2 g, 0.1mol), catechol biborate (59.4g, 0.25mol) and anhydrous potassium acetate (29.4g, 0.3mol) were successively added into the reaction flask , then add 720mL of toluene, stir evenly, then add Pd(PPh3)4 (0.92g, 0.8mmol), then slowly raise the temperature to 110°C under stirring to react, stir for 8-10 hours, the system is black at this time, TLC detection Reaction The reaction is complete. After cooling down to room temperature, 110 mL of water was slowly added dropwise to quench, and after the dropwise addition was completed, the reaction was continued to stir for 4-5 hours. Cool the reaction solution to 0°C, add 10% aqueous hydrochloric acid dropwise to adjust the pH=1, discard the organic layer after layering, add 30% potassium hydroxide to the aqueous layer to adjust the pH=5-6, extract three times with 120 mL of ethyl acetate, add Activated carbon and silica gel are refluxed for decolorization. After fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com