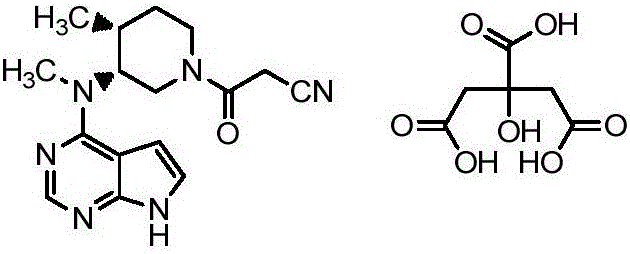
Preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, which is applied in the field of medicine and chemical industry, can solve the problems of difficult control and complete splitting of chiral isomers, low overall yield of chiral side chains, and instability of tert-butyl lithium. , to achieve the effect of reducing equipment cost and operation difficulty, sufficient market supply, and stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of (3R, 4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride
[0048] [Ir(COD)Cl] 2 (0.7g, 1mmol) and (R)-MEO-BiPhep (0.6g, 1mmol) were suspended in 200ml tetrahydrofuran, reacted for 2 hours, then added 1-benzyl-4-methyl-2,6-dihydro- 3-ketopiperidine (20.1 g, 100 mmol), at room temperature, was passed hydrogen gas into the reaction solution, and the pressure was controlled at 5 atm, and the reaction was completed by TLC monitoring. Add saturated brine for washing, dry the organic phase with anhydrous sulfuric acid, concentrate the organic phase, and recrystallize the residue with ethyl acetate to obtain 18.34 g of 1-benzyl-4-methyl-3-ketopiperidine, with a yield of 90.3%. Optical purity 99.0% (HPLC method).
[0049] TiCl 4 (0.2g, 1mmol), NEt 3 (0.1g, 1mmol) was suspended in 200ml tetrahydrofuran, then 1-benzyl-4-methyl-3-ketopiperidine (20.3g, 100mmol) was added, and methylamine solution (7.75g , 100mmol) reacted for 4 hours, add...
Embodiment 2
[0050] Example 2: Synthesis of (3R, 4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride
[0051] [Ir(COD)Cl] 2 (0.7g, 1mmol) and (R)-MEO-BiPhep (0.5g, 0.9mmol) were suspended in 200ml of dichloromethane, reacted for 2 hours, then added 1-benzyl-4-methyl-2,6- Dihydro-3-ketopiperidine (20.1 g, 100 mmol) was controlled at a temperature of 0° C., hydrogen gas was introduced into the reaction solution, and the pressure was controlled at 5 atm. After the reaction was completed, TLC was monitored. Add saturated brine for washing, dry the organic phase with anhydrous sulfuric acid, concentrate the organic phase, and recrystallize the residue with ethyl acetate to obtain 18.55 g of 1-benzyl-4-methyl-3-ketopiperidine, with a yield of 91.3%. Optical purity 99.4% (HPLC method).
[0052] TiCl4 (0.2g, 1mmol), NEt3 (0.09g, 0.9mmol) were suspended in 200ml of dichloromethane, then 1-benzyl-4-methyl-3-ketopiperidine (20.3g, 100mmol) was added, Add methylamine solution (8.5g, ...
Embodiment 3
[0053] Example 3: Synthesis of (3R, 4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride
[0054] [Ir(COD)Cl] 2 (0.7g, 1mmol) and (R)-MEO-BiPhep (0.9g, 1.5mmol) were suspended in 200ml toluene, reacted for 2 hours, then added 1-benzyl-4-methyl-2,6-dihydro -3-ketopiperidine (20.1 g, 100 mmol), temperature controlled at 35° C., hydrogen gas was introduced into the reaction liquid, the pressure was controlled at 5 atm, and TLC was monitored after the reaction was complete. Add saturated brine to wash, dry the organic phase with anhydrous sulfuric acid, concentrate the organic phase, and recrystallize the residue with ethyl acetate to obtain 18.69 g of 1-benzyl-4-methyl-3-ketopiperidine, with a yield of 92.0%. Optical purity 99.1% (HPLC method).
[0055] TiCl 4 (0.2g, 1mmol), NEt 3 (0.15g, 1.5mmol) was suspended in 200ml of toluene, then 1-benzyl-4-methyl-3-ketopiperidine (20.3g, 100mmol) was added, and methylamine solution was added to the reaction solution at a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com