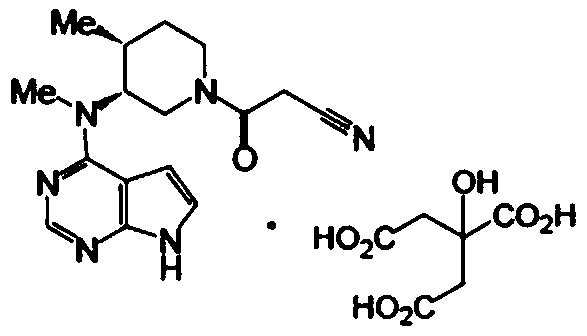
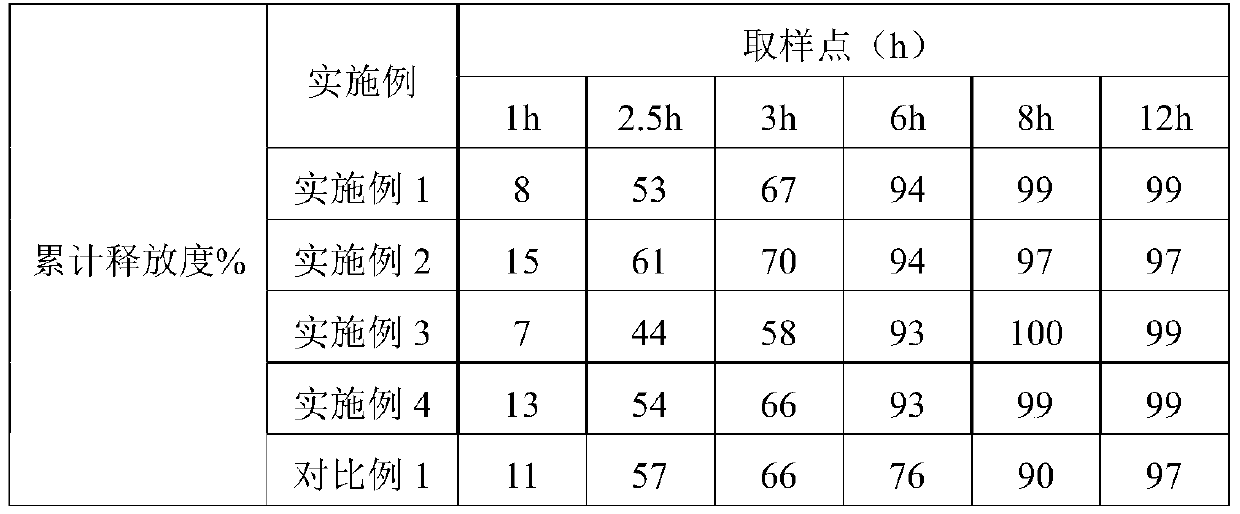
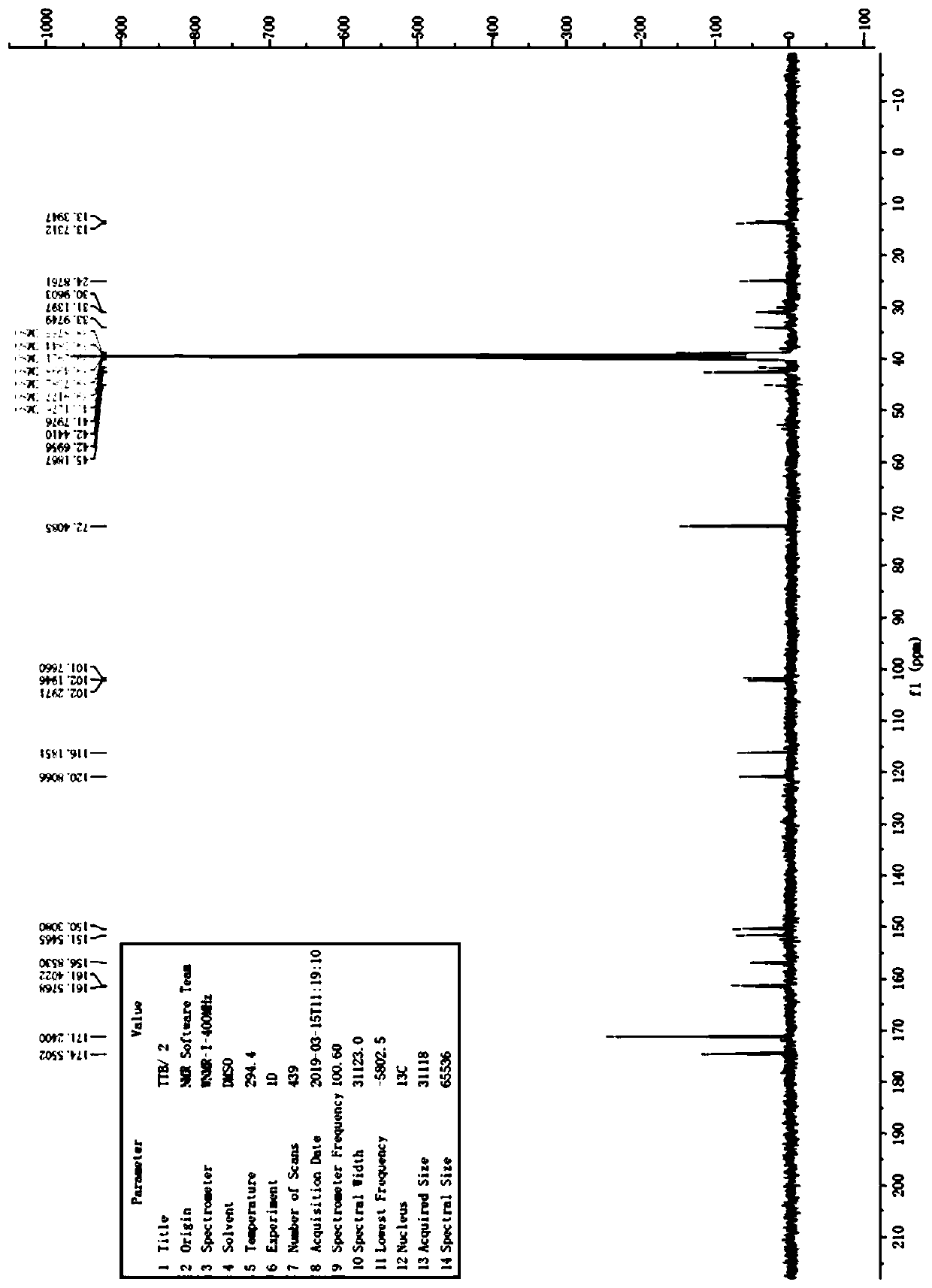
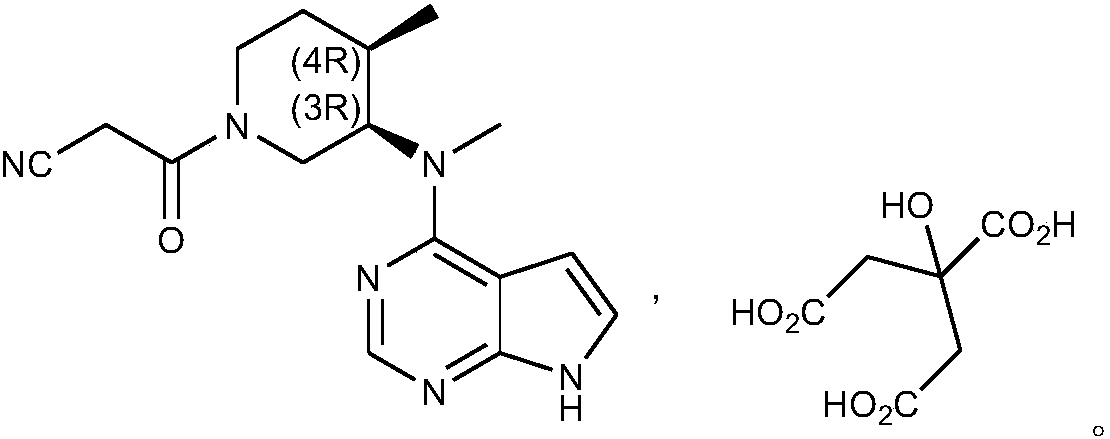
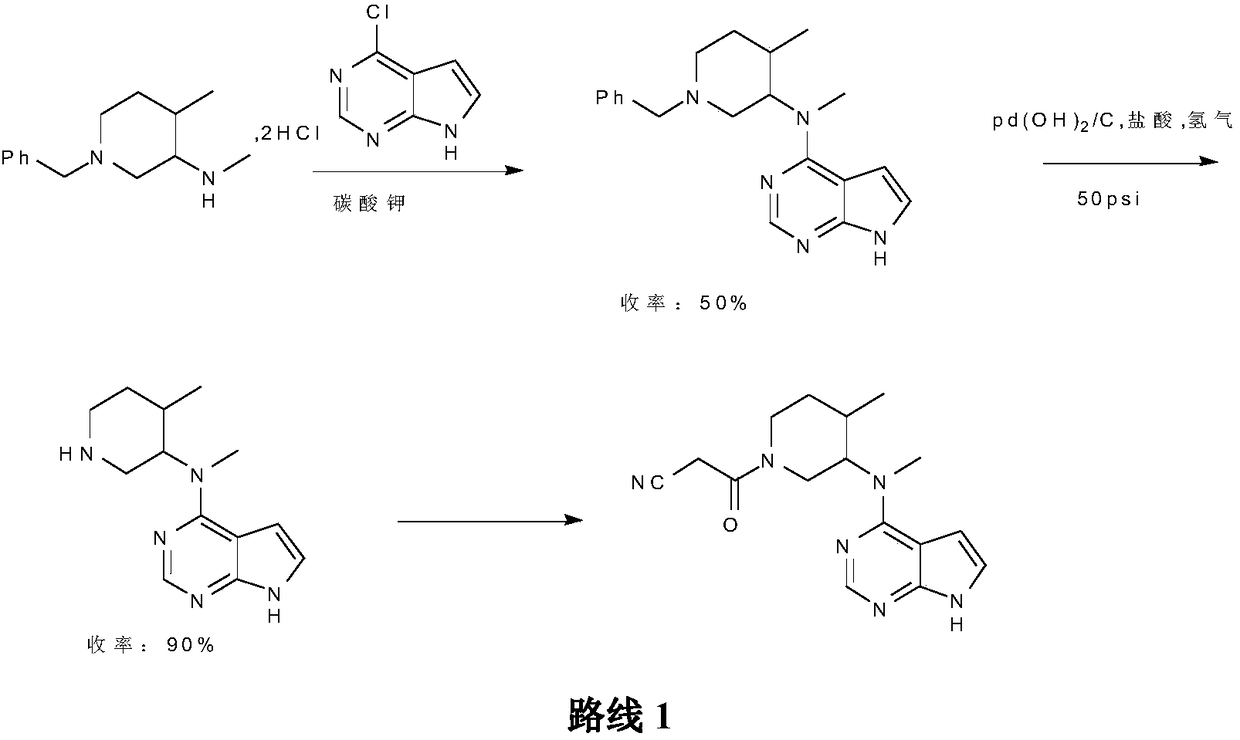
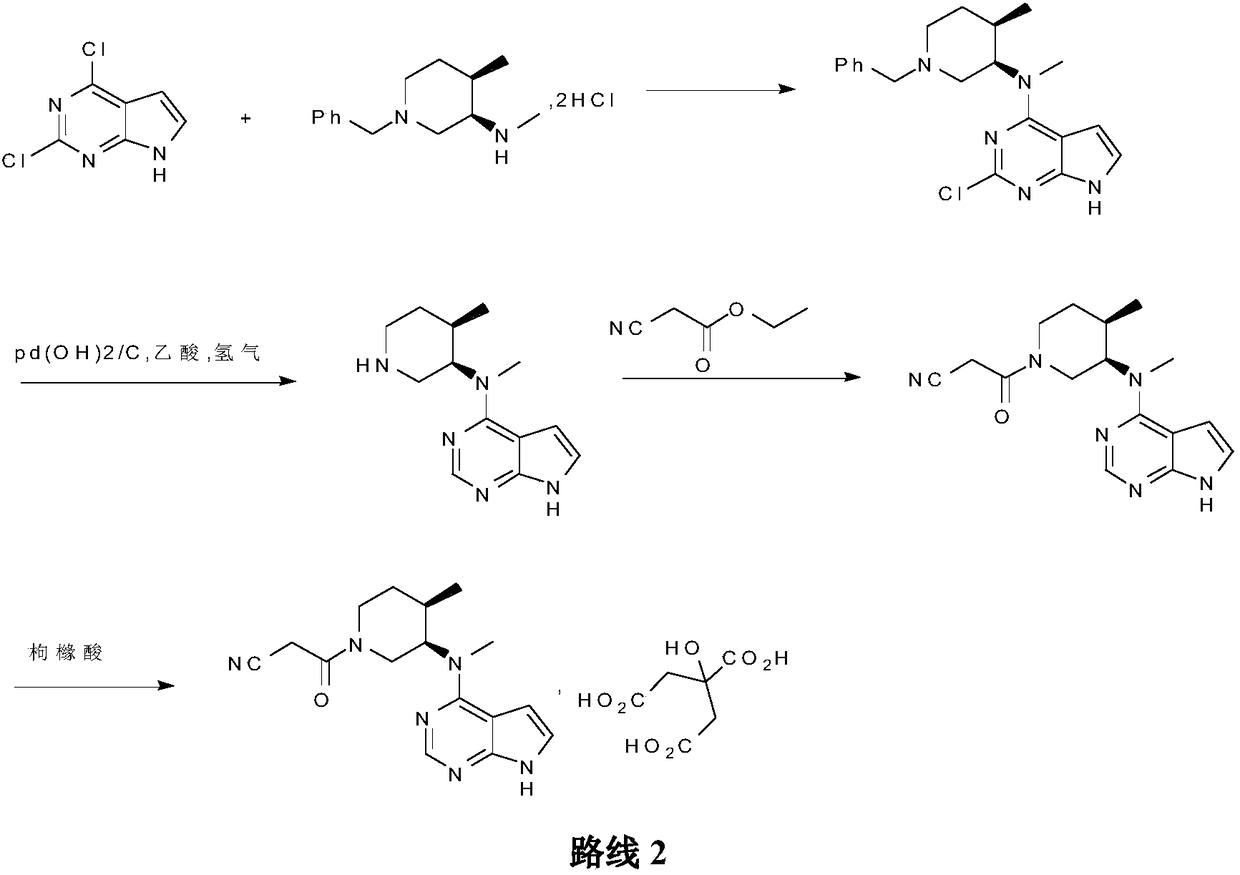
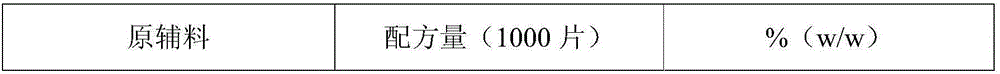Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "TOFACITINIB CITRATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tofacitinib citrate (Xeljanz) is an oral medication prescribed to treat rheumatoid arthritis. Side effects, drug interactions, dosage, and pregnancy safety information should be reviewed prior to taking this medication.
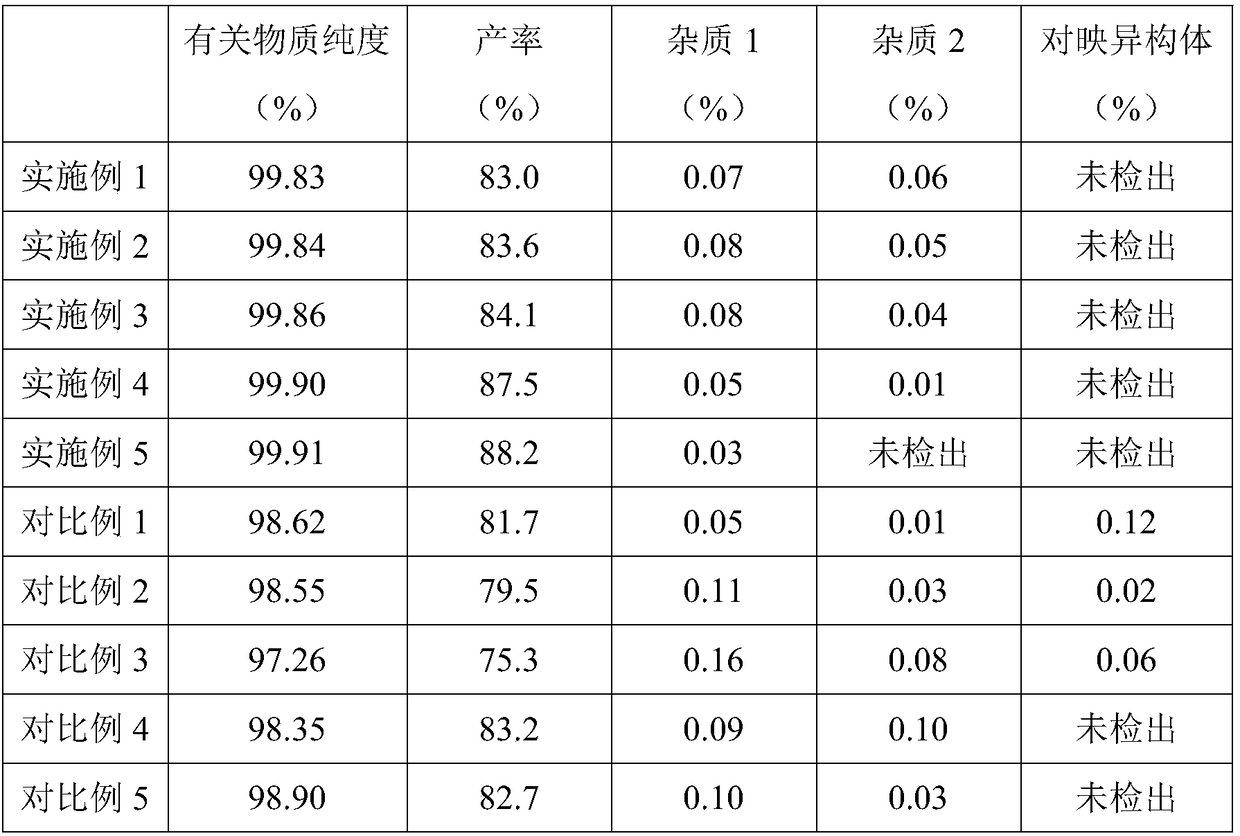
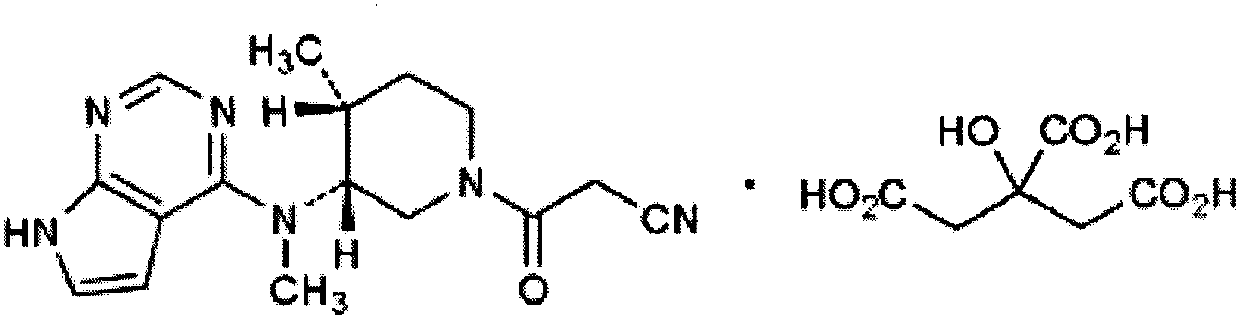
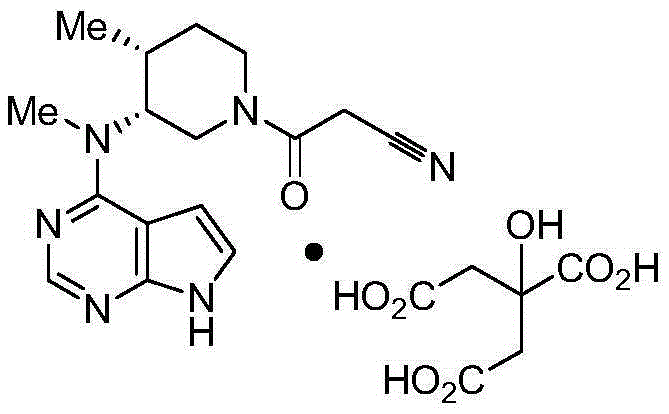
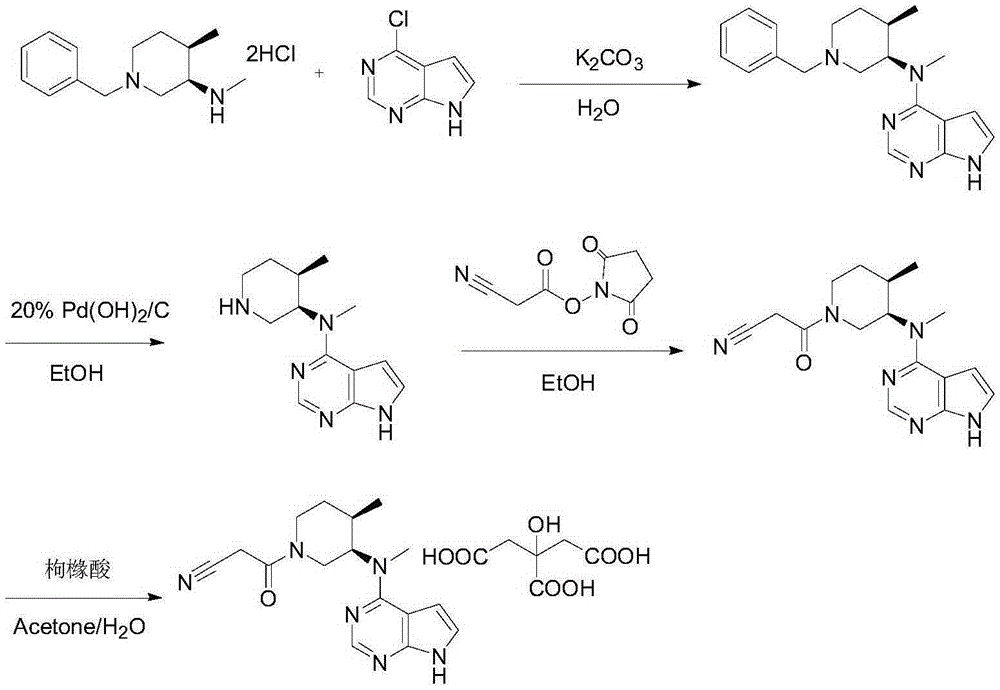
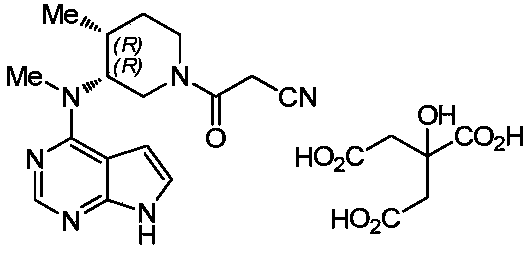
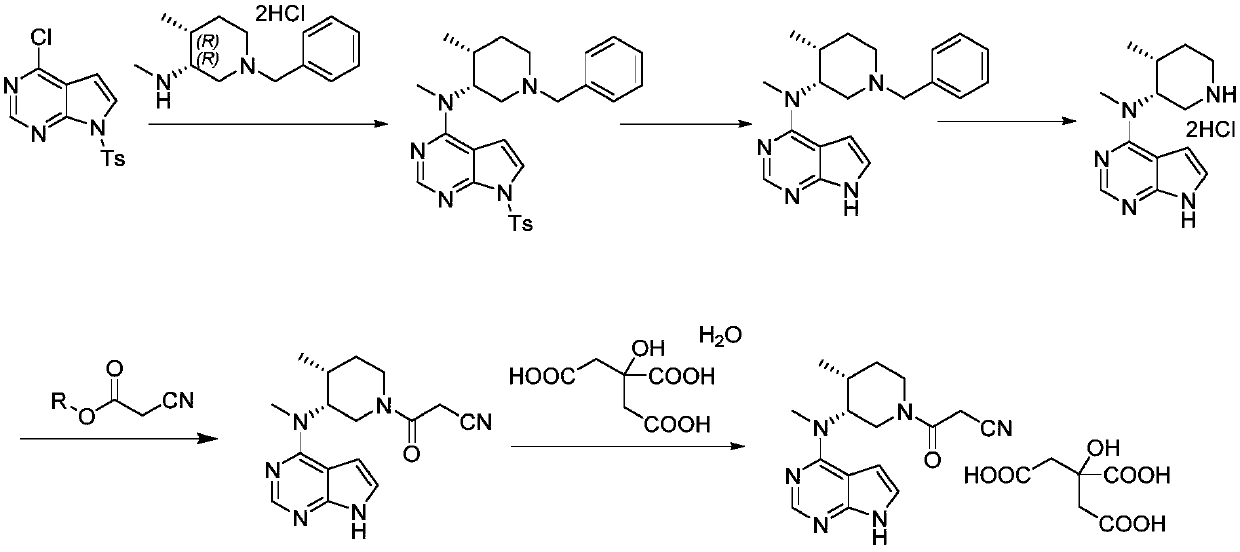
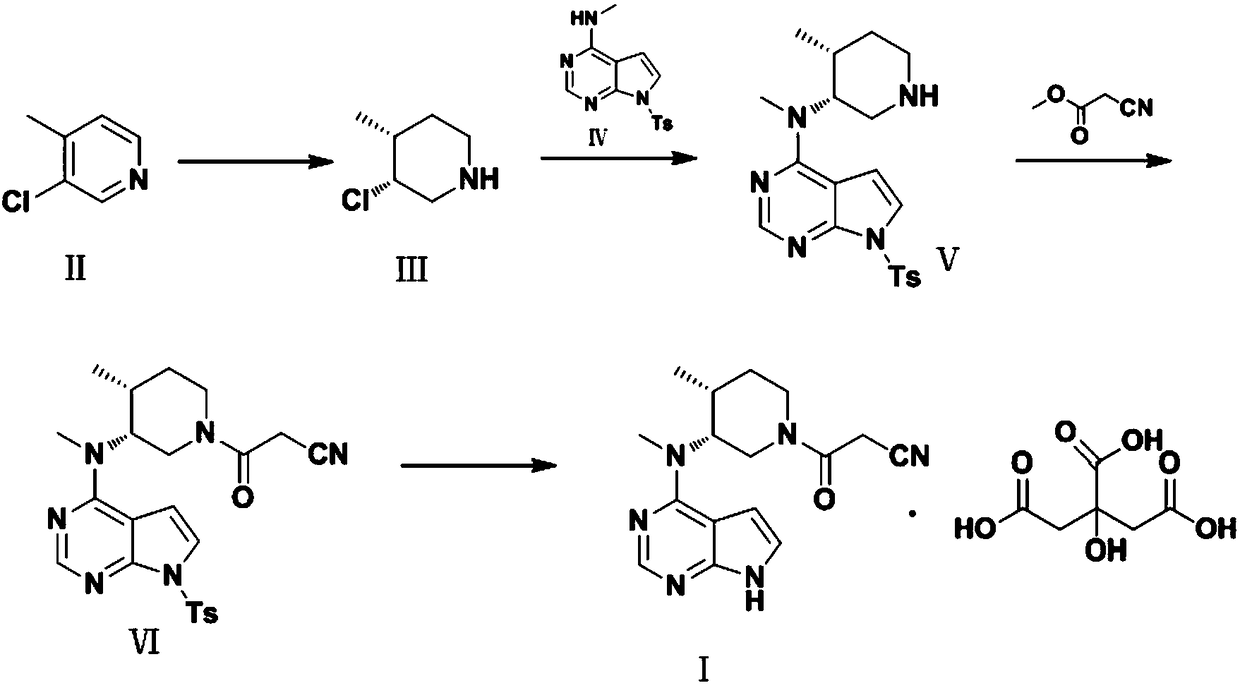
Preparation method of tofacitinib citrate
ActiveCN105884781AEasy to purifyGood stereoselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsCompound (substance)Benzyl group
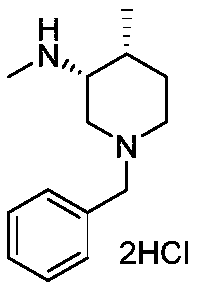
The invention belongs to the field of medicine and chemical engineering and particularly relates to a preparation method of tofacitinib citrate. The method comprises steps as follows: 1-benzyl-4-methyl-2,6-dihydro-3-piperidone taken as a starting material is subjected to an asymmetric reduction reaction, 1-benzyl-4-methyl-3-piperidone is obtained, and (3R,4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride is produced under the action of a chiral catalyst; (3R,4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochlorid and a paratoluensulfonyl chloride protection product 4-chloro-7-(methyl-4-benzenesulfonyl) pyrrolo[2,3-d]pyrimidine of 4-chloropyrrolo[2,3-d]pyrimidine are subjected to a condensation reaction, [(3R,4R)]-1-benzyl-4-methyl-piperidine-3-yl]-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)-amine is obtained through deprotection, and tofacitinib citrate is obtained through debenzylation protection, an acylation reaction and citric acid salifying. The process route is short, the process cycle is short, chiral synthesis is performed by means of a catalyst, the product purity is improved, the cost is reduced, the yield is high, and the operation is simple and convenient.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Content determining and related substance detection method for tofacitinib citrate
ActiveCN104459004AImprove the detection rateHigh precisionComponent separationQuality controlRepeatability
Owner:NANJING CORE TECH CO LTD
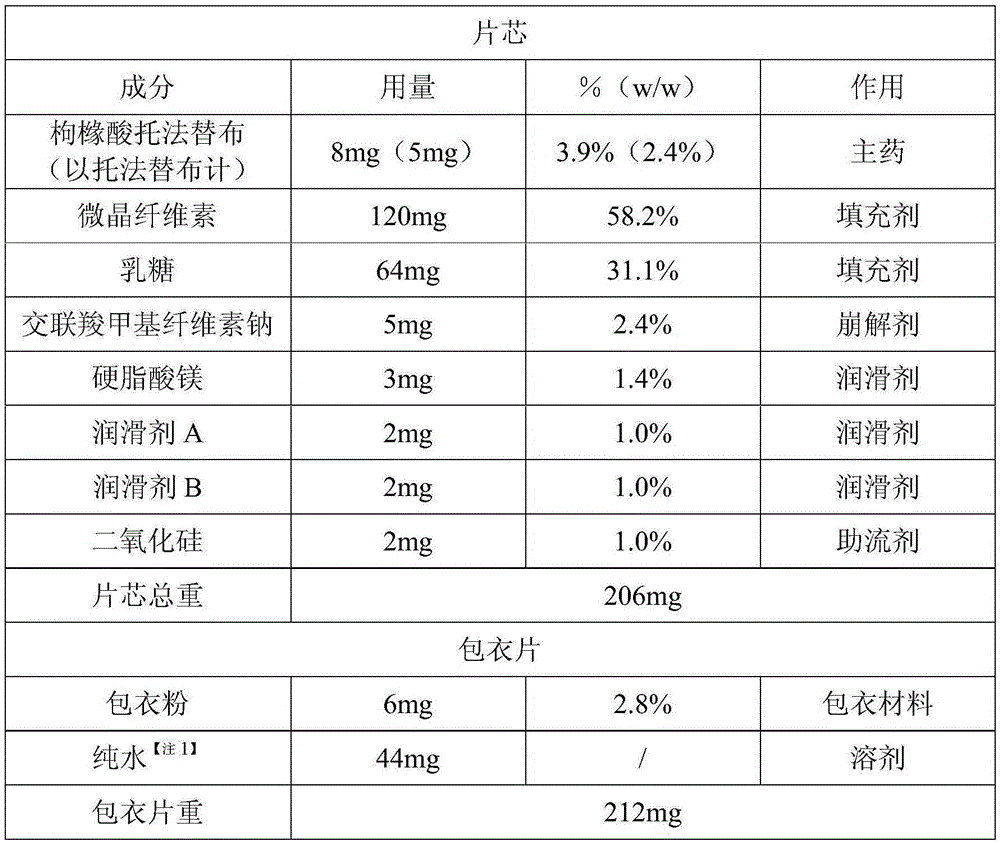
Tofacitinib citrate tablets and preparation method thereof
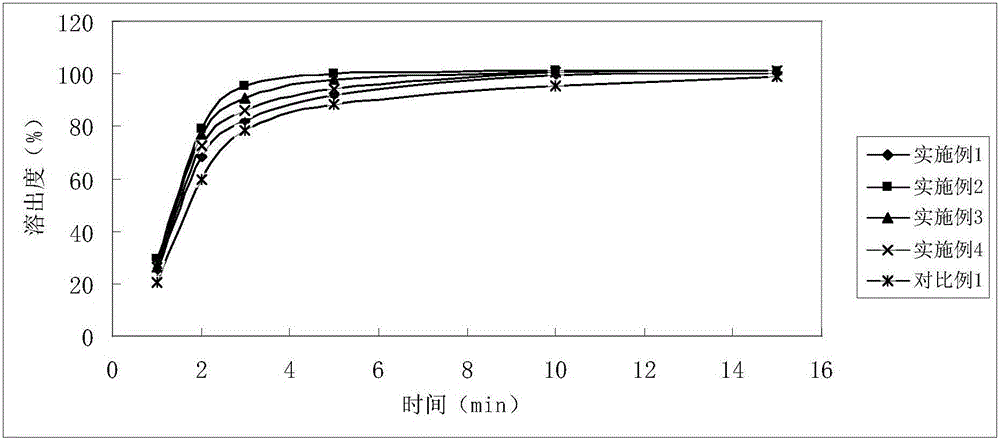
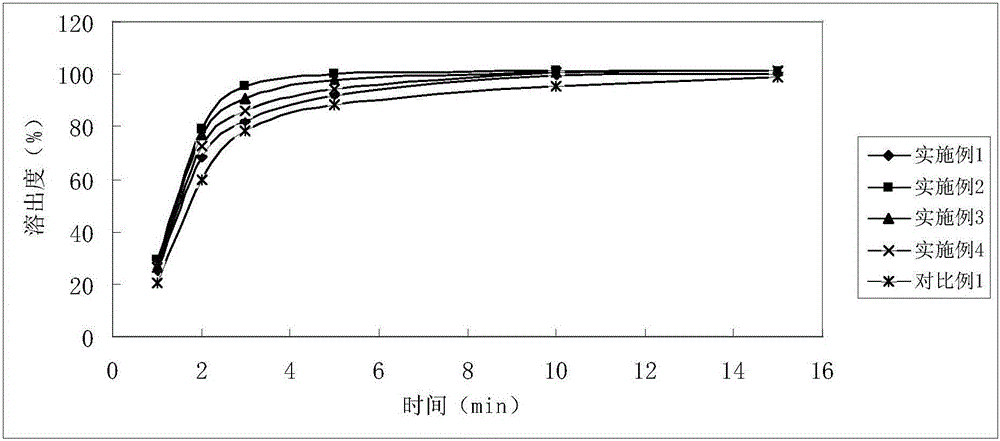
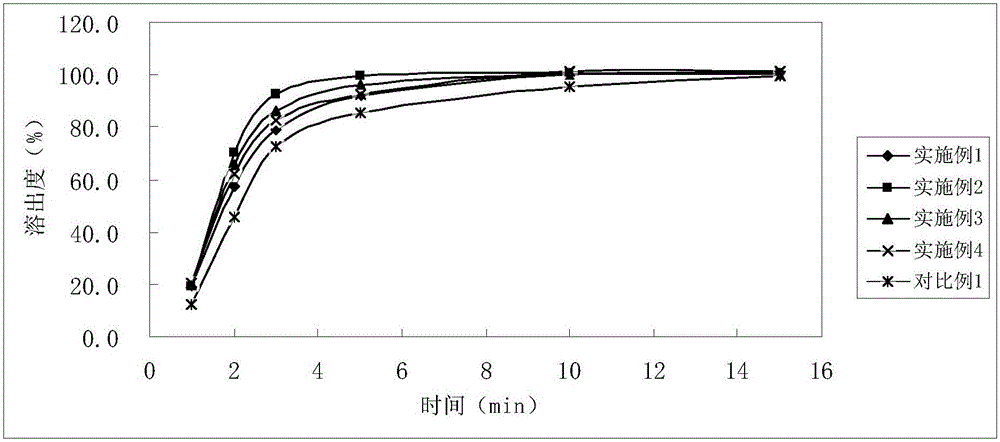
InactiveCN105878202AGood content uniformityDissolution rate is fastOrganic active ingredientsAntipyreticFiller ExcipientLactose
The invention discloses Tofacitinib citrate tablets composed of Tofacitinib citrate and pharmaceutically acceptable auxiliary materials. The auxiliary materials comprise filler, disintegrating agent, flow aid and coating materials. The filler is microcrystalline cellulose lactose premix with the model of Celactose80, and the weight ratio of the Tofacitinib citrate to the microcrystalline cellulose lactose premix with the model of Celactose80 is 1:5-25. The invention further discloses a preparation method. The Tofacitinib citrate tablets has high content uniformity and have the advantages of being high in dissolving-out speed, stable in chemical property and the like.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
Preparation method for amorphous tofacitinib citrate
InactiveCN103073552AImprove solubilityImprove stabilityOrganic chemistryOrganic solventTOFACITINIB CITRATE
Owner:BEIJING PHARMA GRP CO LTD
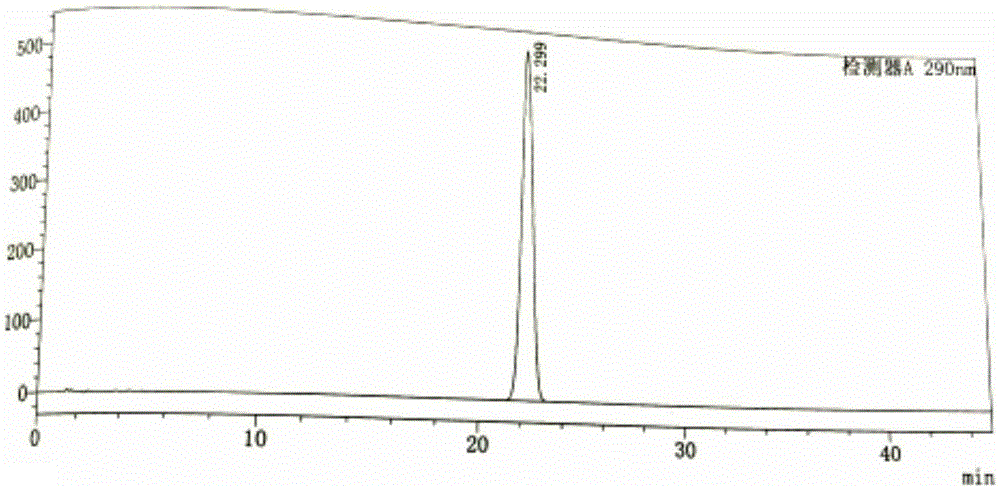
Determination method for content of tofacitinib citrate and related substances of tofacitinib citrate by reversed phase high-performance liquid chromatography
The invention discloses a determination method for the content of tofacitinib citrate and related substances of the tofacitinib citrate by reversed phase high-performance liquid chromatography. The determination method is characterized in that octadecylsilane chemically bonded silica is taken as filler; 0.02mol / L potassium dihydrogen phosphate solution and acetonitrile with volume ratio of 83:1 are taken as a mobile phase to perform isocratic elution, and the flow speed is 1.0ml / min; the 0.02mol / L potassium dihydrogen phosphate solution contains trimethylamine with mass concentration of 0.2 percent, and pH value is adjusted to 5.2 by using phosphoric acid; the detection wavelength is 290nm, and the column temperature is 30DEG C. According to the determination method disclosed by the invention, by adopting the isocratic elution, requirements on an instrument are reduced, as well as stable pressure and accurate and reliable experimental result are obtained; meanwhile, by adopting the method disclosed by the invention, the concentration of linear range is improved, and the preparation for a test solution during experiment is facilitated.
Owner:NINGBO LIWAH PHARM CO LTD
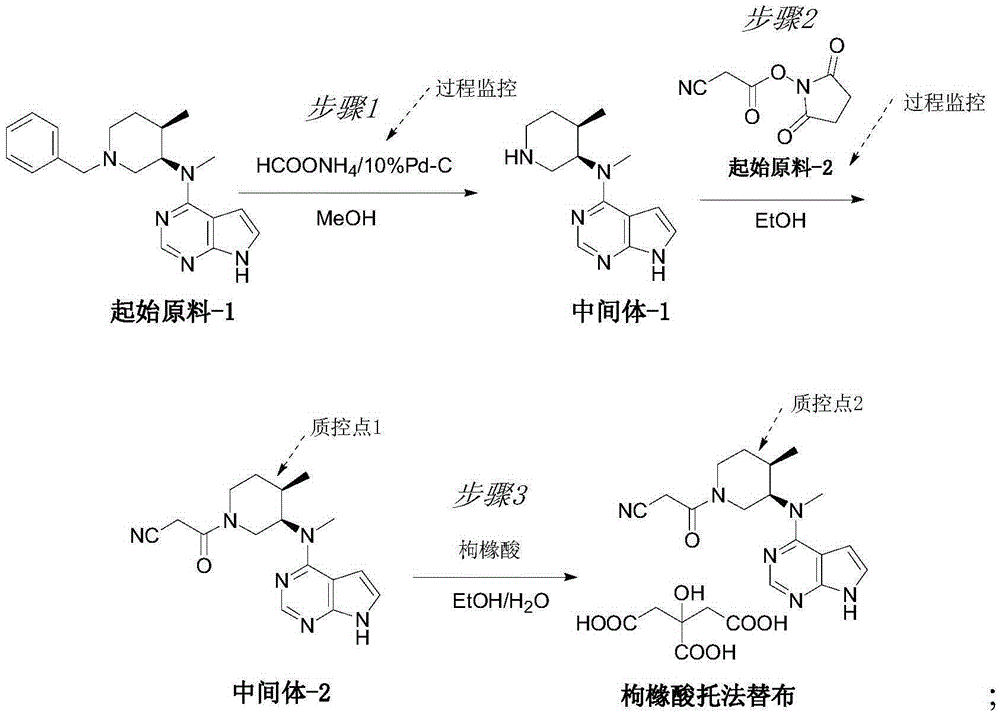
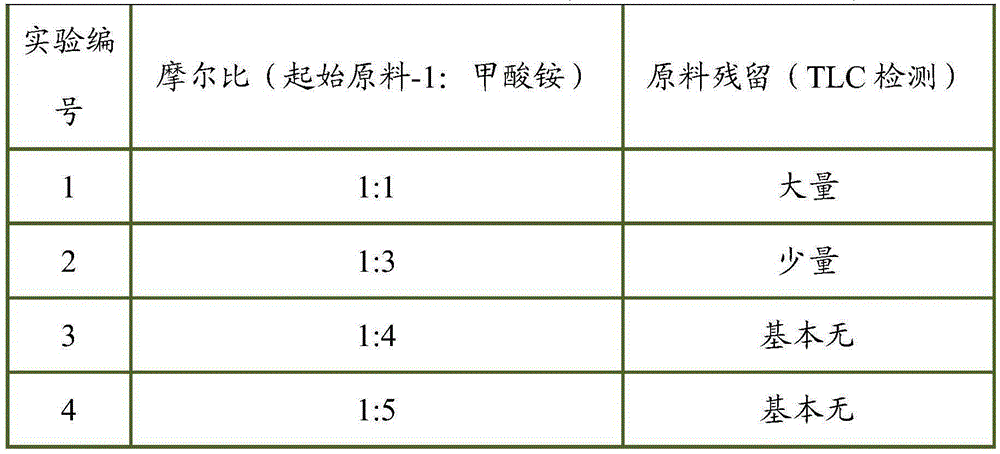
Novel synthetic process of tofacitinib citrate
InactiveCN105348287AReasonable routingMild reaction conditionsOrganic chemistryPalladium on carbonChemical synthesis
The invention discloses a novel synthetic process of tofacitinib citrate. The novel synthetic process comprises the steps that 1, an initial raw material-1, 10% of palladium on carbon, absolute methanol and ammonium formate are mixed in a reaction container, and reacting is carried out to obtain a midbody-1; 2, the midbody-1 prepared in the step 1 is dissolved into absolute ethyl alcohol, an initial raw material-2 is added, reacting is carried out at the reaction temperature of 20 DEG C to 50 DEG C, reaction liquid is purified to obtain a crude midbody-2 after reacting is finished, and the crude midbody-2 is refined to obtain a refined midbody-2; 3, the refined midbody-2 is subjected to heating reflux and dissolved clarification through absolute ethyl alcohol, a citric acid water solution is dropwise added, and reacting continues to be carried out at the temperature of 50 DEG C to 90 DEG C; then the mixture is slowly cooled to 20 DEG C to 45 DEG C, and stirring and devitrification are carried out; crystals are filtered and washed with ethyl alcohol, drying is carried out under reduced pressure at the temperature of 40 DEG C to 60 DEG C, and white crude tofacitinib citrate is obtained. The chemical synthetic process is more reasonable in route, and the reaction conditions are milder.
Owner:NINGBO LIWAH PHARM CO LTD
Preparation method of medicinal crystal form tofacitinib citrate
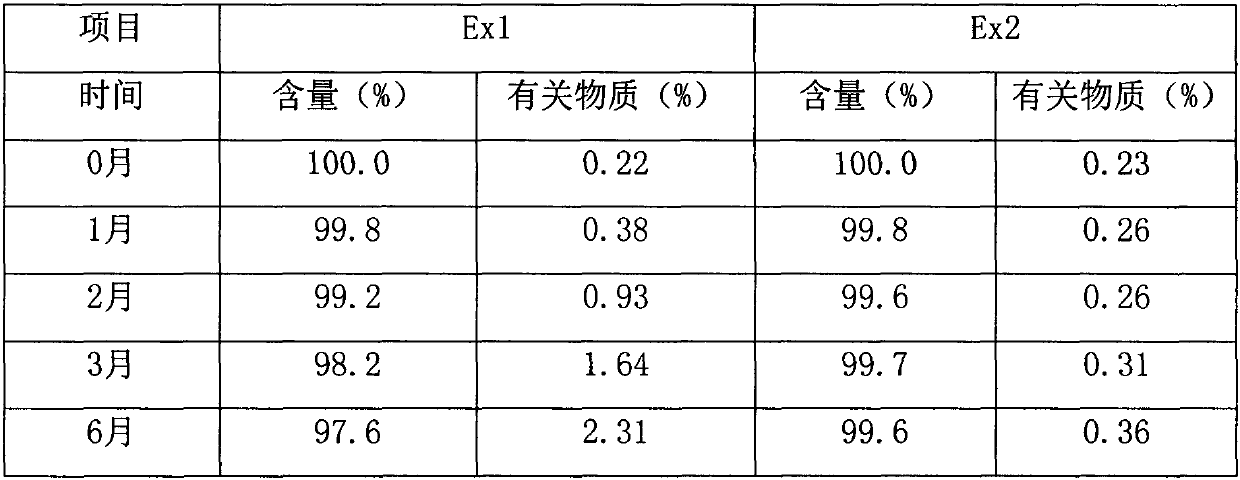
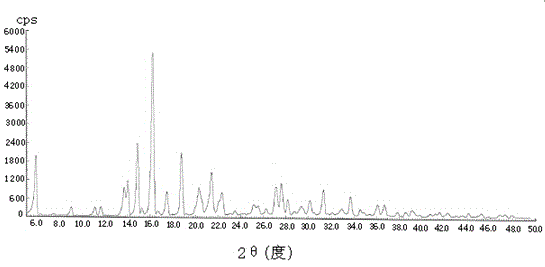
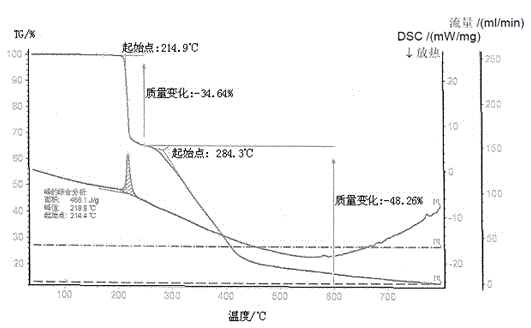
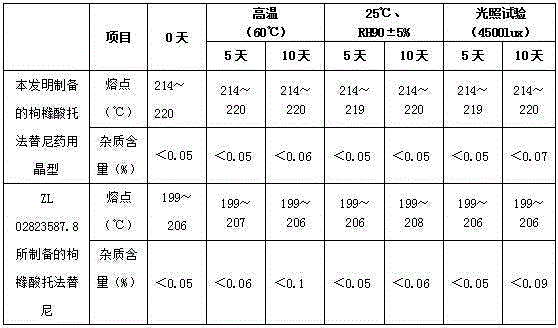
The invention provides a preparation method of medicinal crystal form tofacitinib citrate. The preparation method comprises the following steps of 1, taking a tofacitinib citrate raw material, pumping a solvent into the raw material in vacuum, heating the mixture for dissolution, and carrying out thermal insulation stirring for 1-2h, 2, carrying out slow cooling to 0-50 DEG C, and carrying out thermal insulation stirring for 4-10h for crystallization, and 3, carrying out filtration and drying to obtain the medicinal crystal form tofacitinib citrate. Through strict control of a crystallization temperature, crystallization time and a use amount of a solvent in the process, the preparation method greatly improves a yield and purity of tofacitinib citrate, the highest yield is 90% and purity is more than 99.7% The preparation method has less total impurities and has all single impurity contents less than 0.1%. A powdery X-ray diffractometer test proves that the finished product obtained by the preparation method has a single and stable crystal form and satisfies medicinal-grade bulk drug requirements.
Owner:合肥远志医药科技开发有限公司
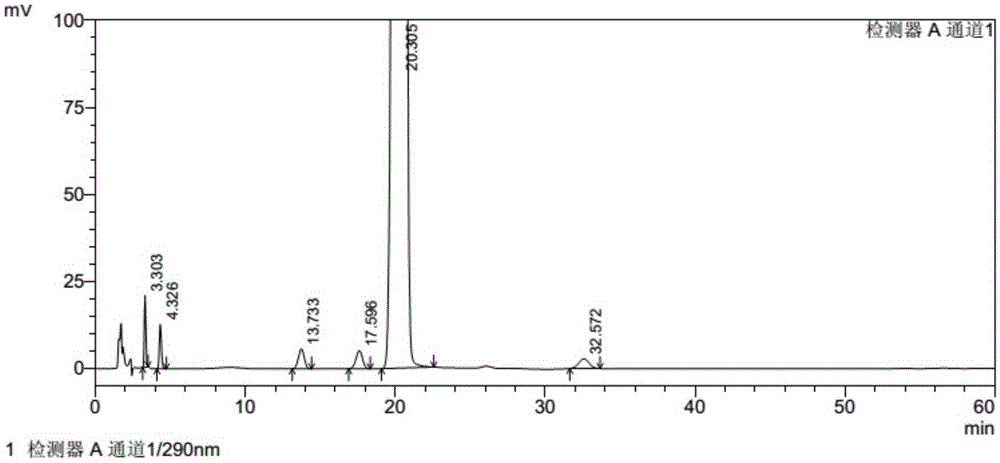
Method for separating and measuring tofacitinib citrate and optical isomer of tofacitinib citrate by adopting liquid chromatography
InactiveCN104678001AEffective separation assayQuality is easy to controlComponent separationCelluloseAlcohol
The invention relates to a method for separating and measuring tofacitinib citrate and an optical isomer of the tofacitinib citrate by adopting a liquid chromatography. The method is characterized in that a chiral chromatographic column taking tri(3,5-dichlorophenyl)-carbamate cellulose or tri(3,5-dimethyl phenyl)-carbamate cellulose as filler is adopted, and a normal hexane-low alcohol-amine solution is taken as a mobile phone, wherein amine is diethylamine or triethylamine, the concentration of the amine is 0.05%-0.5%V / V of that of normal hexane, and the volume ratio of the normal hexane to low alcohol is (55 to 45) to (85 to 15). The method is stable in lower base line, relatively good in main peak shape and relatively high in detection limit, so that the mass of the tofacitinib citrate can be accurately controlled.
Owner:CHONGQING PHARMA RES INST
Preparation method of tofacitinib citrate
ActiveCN104292231AOrganic chemistry methodsBulk chemical productionAfter treatment4-methylpiperidine
The invention relates to a method for preparing tofacitinib citrate. The method comprises the following steps: by taking N-methyl-N-((3R, 4R)-1-benzyl-4-methylpiperidine-3-yl)-7H-pyrrolo (2, 3-d) pyrimidin-4-amine as a raw material, performing hydrogenation to remove an amino protecting group, performing amidation reaction and salt-forming reaction and finally drying to obtain tofacitinib citrate. In the preparation process, after-treatment is not required, the separation of a target intermediate is not required, and only reactants need to be added sequentially. The preparation method of tofacitinib citrate provided by the invention has the advantages of simplicity in operation, high yield, mild conditions and the like, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
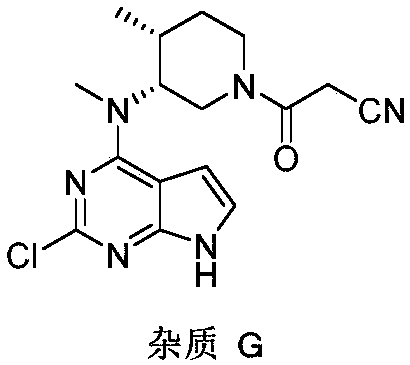
A tofacitinib citrate purification method
ActiveCN108948020AEfficient removalSimple and fast operationOrganic chemistry methodsPurification methodsSolvent
The invention belongs to the field of medicine synthesis and provides a tofacitinib citrate purification method which can effectively remove an impurity G. The method includes adding a tofacitinib citrate crude product to be purified into a mixed solvent, stirring and heating the mixture until the crude product is fully dissolved, cooling the mixture for crystallization, and performing filtrationand vacuum drying to obtain a purified tofacitinib citrate product. The method is simple to operate and suitable for industrial scale-up production, and has obvious impurity removing effects and a high yield.
Owner:NANJING CHIA TAI TIANQING PHARMA
Preparation methods of tofacitinib citrate intermediate and tofacitinib citrate
InactiveCN110668995AImprove refining effectControl generationOrganic chemistryPtru catalystBenzyl chloride
The invention discloses preparation methods of a tofacitinib citrate intermediate and tofacitinib citrate. The preparation method of the tofacitinib citrate intermediate comprises: preparing N-(1-benzyl-4-methyl-1,2,5,6-tetrahydropiperidine-3-yl)acetamide by using 3-amino-4-methyl-pyridine, acetyl chloride, benzyl chloride and sodium borohydride as raw materials; preparing 1-benzyl-N,4-dimethylpiperidine-3-amine by using the N-(1-benzyl-4-methyl-1,2,5,6-tetrahydropiperidine-3-yl)acetamide, hydrochloric acid, methylamine and sodium borohydride as raw materials; and carrying out resolution and dissociation on the 1-benzyl-N,4-dimethylpiperidine-3-amine, and carrying out salt forming with hydrochloric acid to obtain the product. The invention provides the new tofacitinib citrate intermediatepreparation method, wherein the use amount of the catalytic hydrogenation catalyst is reduced in the preparation process of tofacitinib citrate so as to reduce the cost, and the generation of N-alkylated impurities can be well controlled by adopting the isopropanol / water mixed solvent.
Owner:江苏海悦康医药科技有限公司
Preparation method of tofacitinib citrate
The invention relates to a preparation method of tofacitinib citrate, in particular to high-yield synthesis of tofacitinib citrate. The tofacitinib citrate is synthesized from raw materials including4-chloro-7-pyrrolo[2,3-d]pyrimidine, (BOC)2O, (3R,4R)-(1-benzyl-4-methylpiperidine-3-yl)methylamine-L-di-p-toluoyl tartrate, Pd / C, cyanoacetic acid and citric acid through six steps including an aminoprotection reaction, an amination reaction, a debenzylation reaction, a condensation reaction, a deprotection reaction and a salt forming reaction. The synthesis route provides the preparation methodof tofacitinib citrate, and the preparation method is high in yield, low in cost, easy to operate and suitable for industrialization.
Owner:南京法恩化学有限公司
Preparation method of tofacitinib citrate starting material
InactiveCN107337676AMeet the needs of industrial productionReduce manufacturing costOrganic chemistryPyridiniumSynthesis methods
The invention discloses a synthesis method of a tofacitinib citrate starting material N-((3R, 4R)-4-methyl-1-benzyl-3-piperidyl)-N-methyl-7-tolylsulfonyl-7H-pyrrolo[2,3-D]pyrimidine-4-amine(I). The method comprises the specific steps that 4-methylpyridine is adopted as the starting material to be subjected to nucleophilic substitution with benzyl chloride to obtain 4-methyl-1-benzyl-pyridinium chloride, a reduction reaction is performed under the effect of sodium borohydride, a hydroboration-oxidation reaction is performed, hydroxyl oxidation is performed, two chiral centers are introduced in a reductive amination stereoselectivity mode, splitting is performed through cheap chiral acid (L-DTTA) easy to obtain to obtain an optically pure intermediate body (3R, 4R)-(1-benzyl-4-methyl-piperidine-3-yl)-methyl amine, and finally, the intermediate body and 4-chloropyr are condensed to obtain the tofacitinib citrate starting material. The whole method is easy to implement and low in cost, raw materials are easy to obtain, and aftertreatment is easy. The formula is shown in the description.
Owner:JIANGSU QINGJIANG PHARMA
New method for preparing tofacitinib citrate crystal-form A
InactiveCN104774206ASuitable for industrialized mass productionEasy to operateCarboxylic acid salt preparationTOFACITINIB CITRATEChemistry
The invention belongs to the field of medicine, and in particular, relates to a new method for preparing a tofacitinib citrate crystal-form A. The method has the advantages of being simple to operate, good in reproducibility, high in yield, suitable for industrialized mass production and the like, and overcomes the problems that a conventional method for preparing a tofacitinib citrate crystal form is poor in reproducibility, low in yield, not suitable for industrialized production and the like.
Owner:JIANGSU CAREFREE PHARM CO LTD
Refining method of tofacitinib citrate
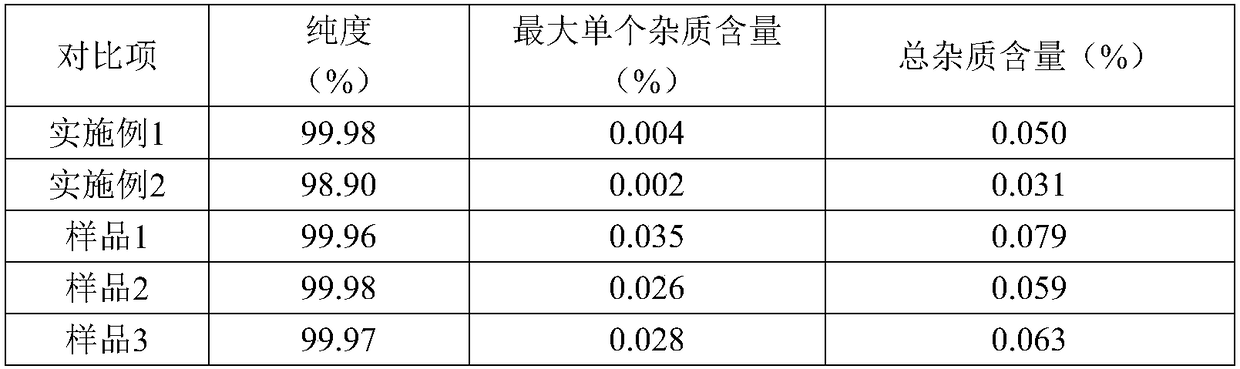
The invention discloses a refining method of tofacitinib citrate. The refining method comprises the following steps: (1) dissolving a tofacitinib citrate coarse product into a mixed solvent composed of methanol and butanone, and controlling the temperature at 45 to 50 DEG C till the tofacitinib citrate is completely dissolved; (2) adding active carbon for decoloration, filtering the solution, cooling filtrate at a certain cooling rate till the temperature is -10 to 0 DEG C, separating out crystals, and growing the crystals; (3) filtering the solution, washing filtered solids with methanol, anddrying the solids in vacuum to obtain the refined tofacitinib citrate. According to the refining method disclosed by the invention, the purity of the obtained tofacitinib citrate can be up to 99.9 percent or above, and the content of total impurities and the content of a single impurity are respectively controlled within 0.1 percent and 0.05 percent; the quality of a product is obviously improved; furthermore, the refining process is easy to operate and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD +2
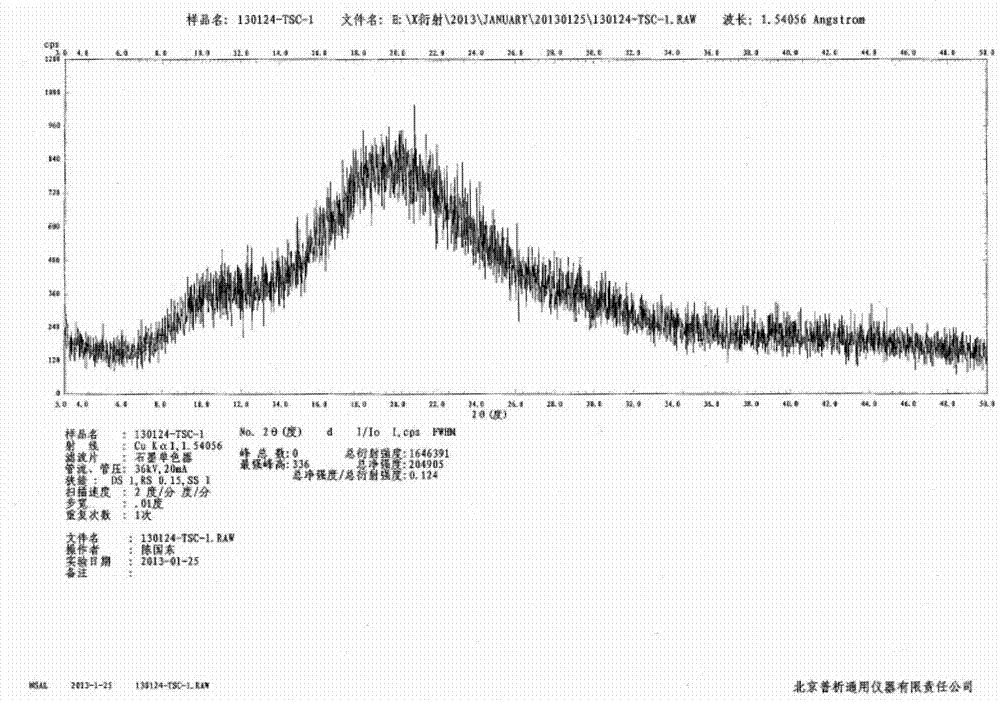
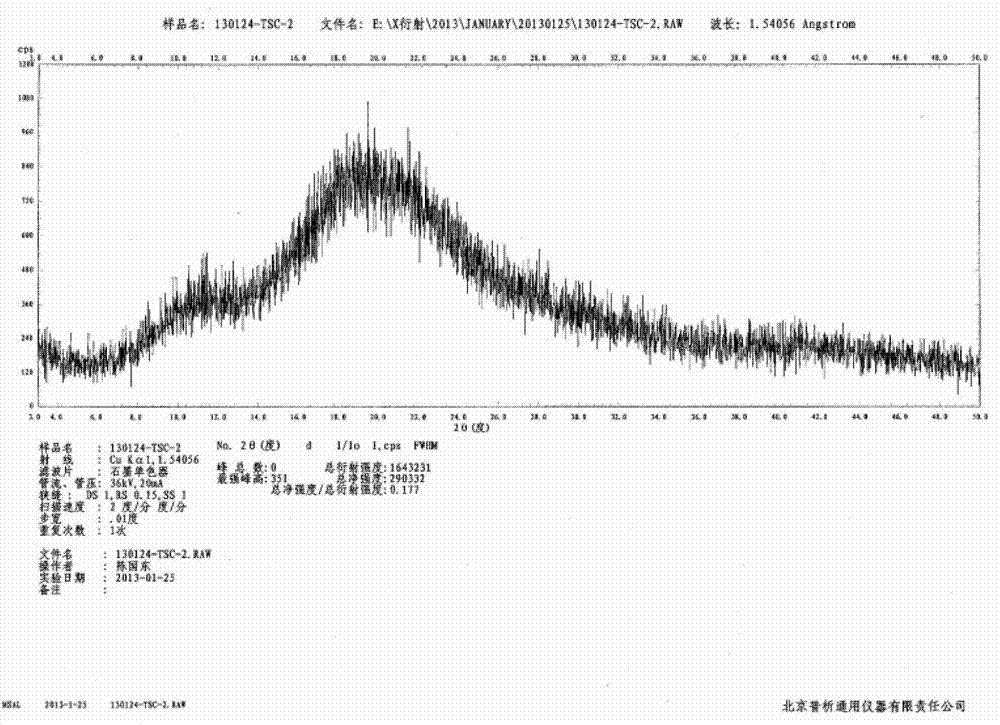
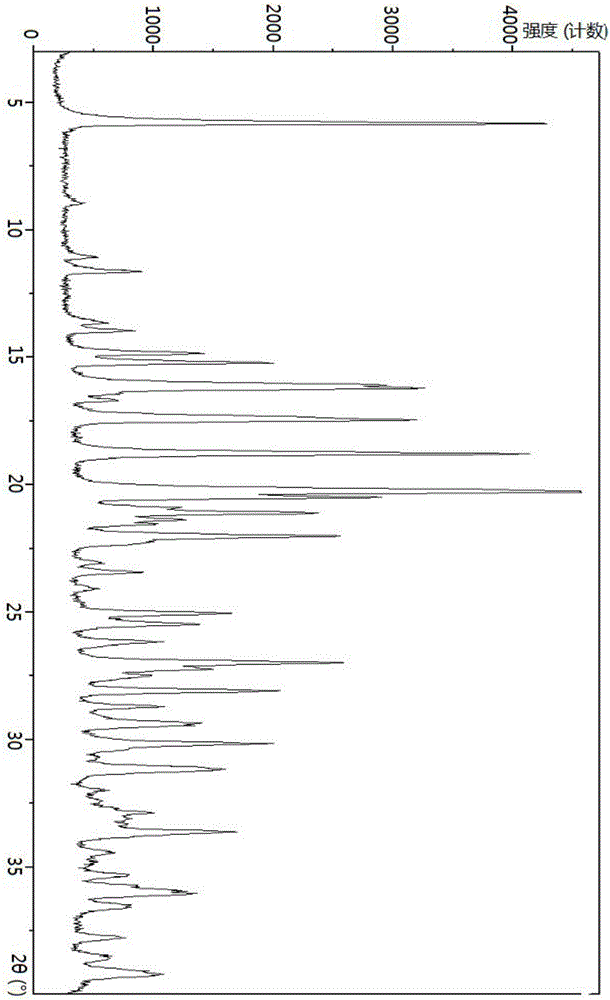
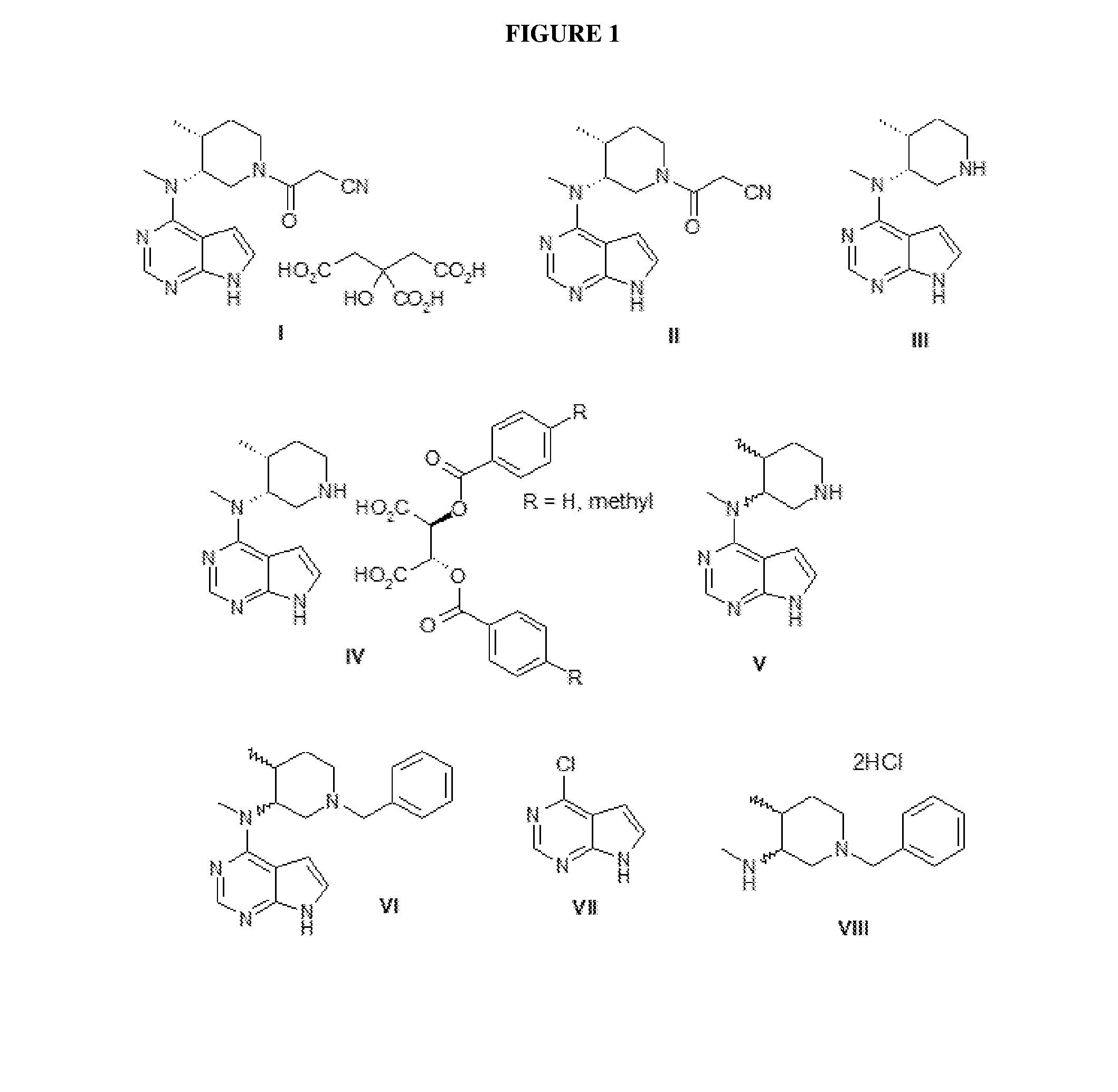
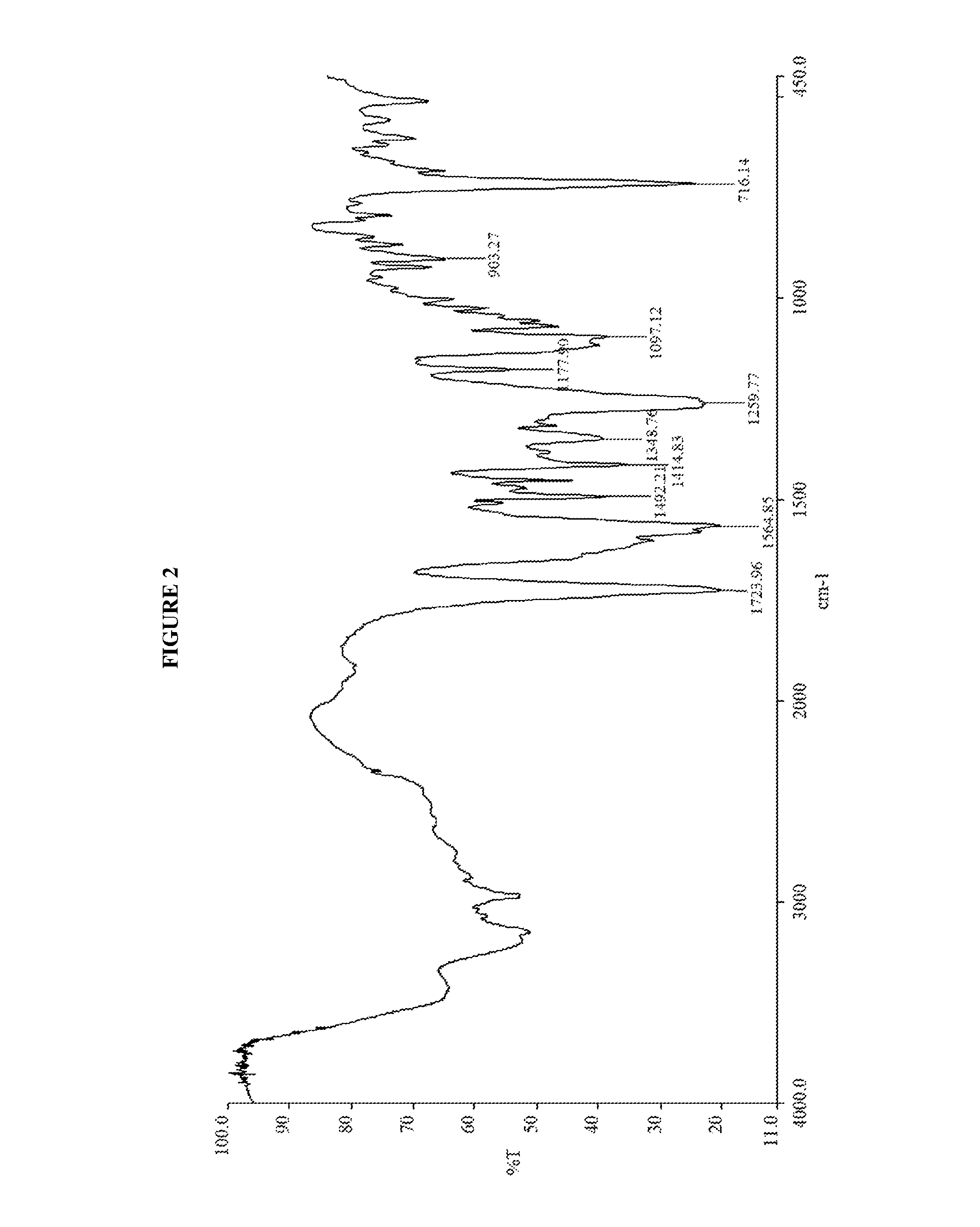
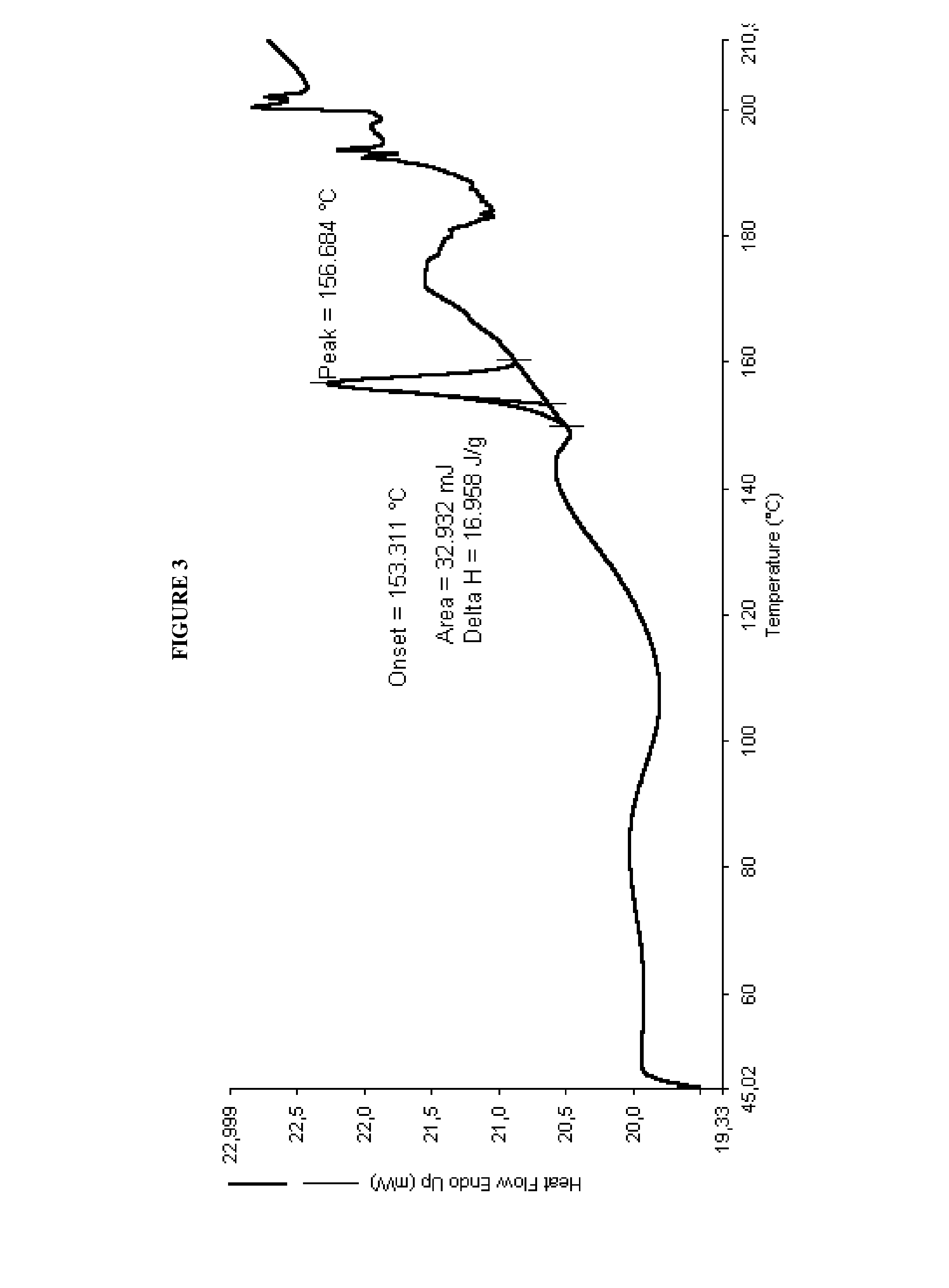
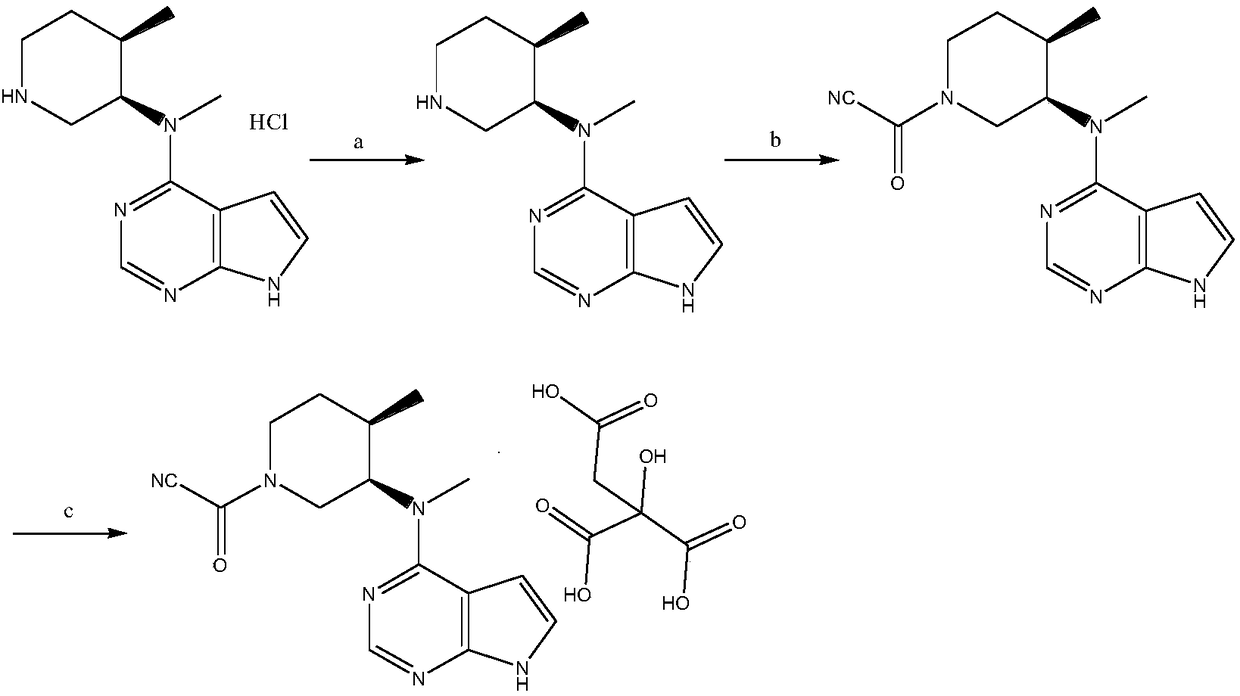
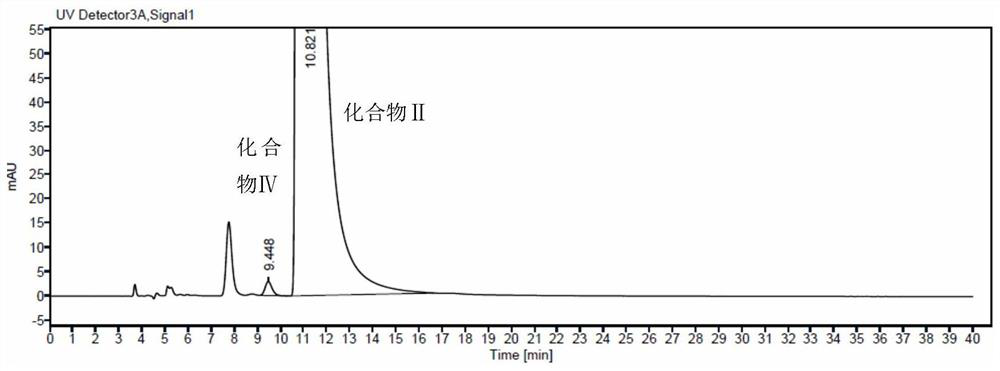
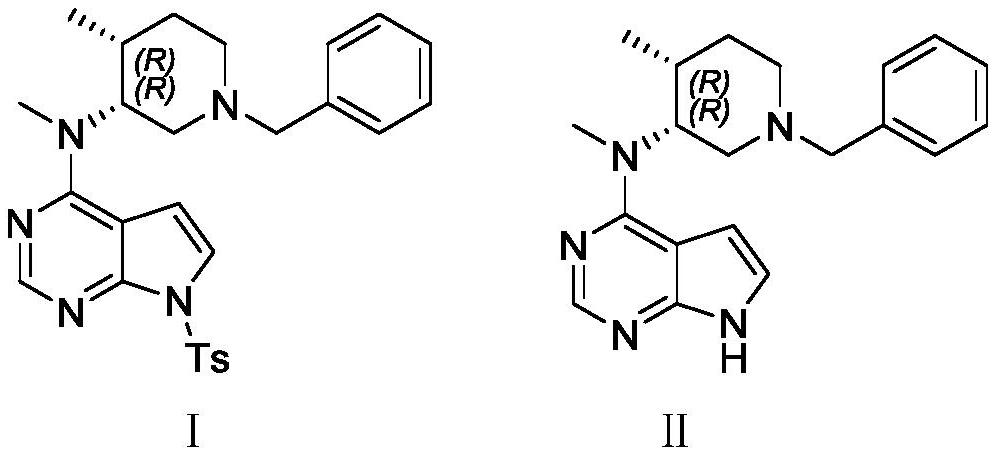
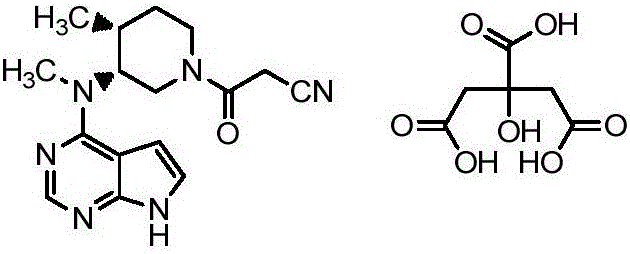
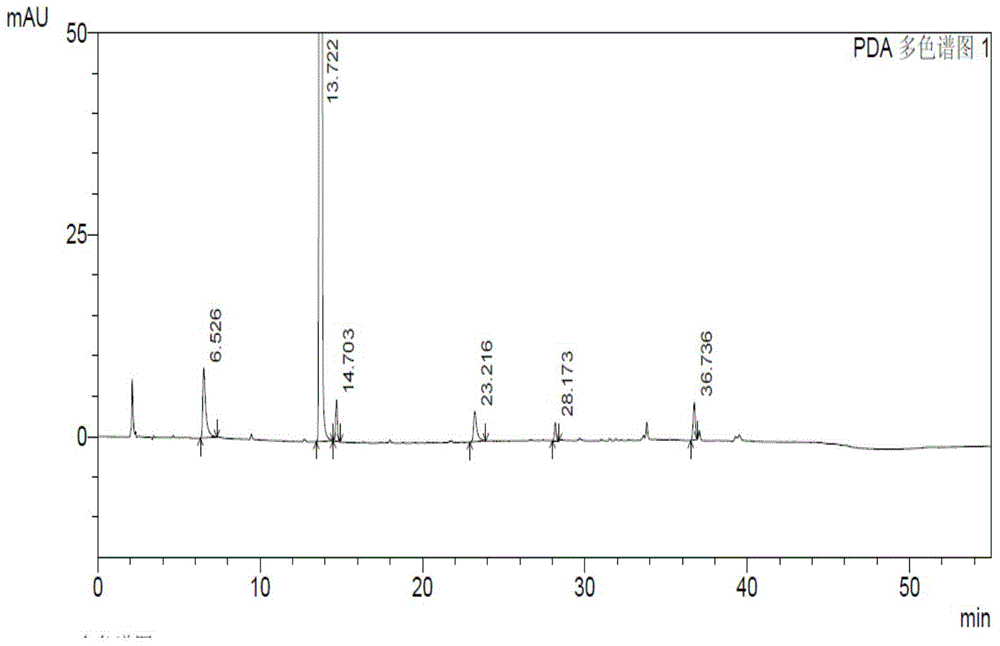
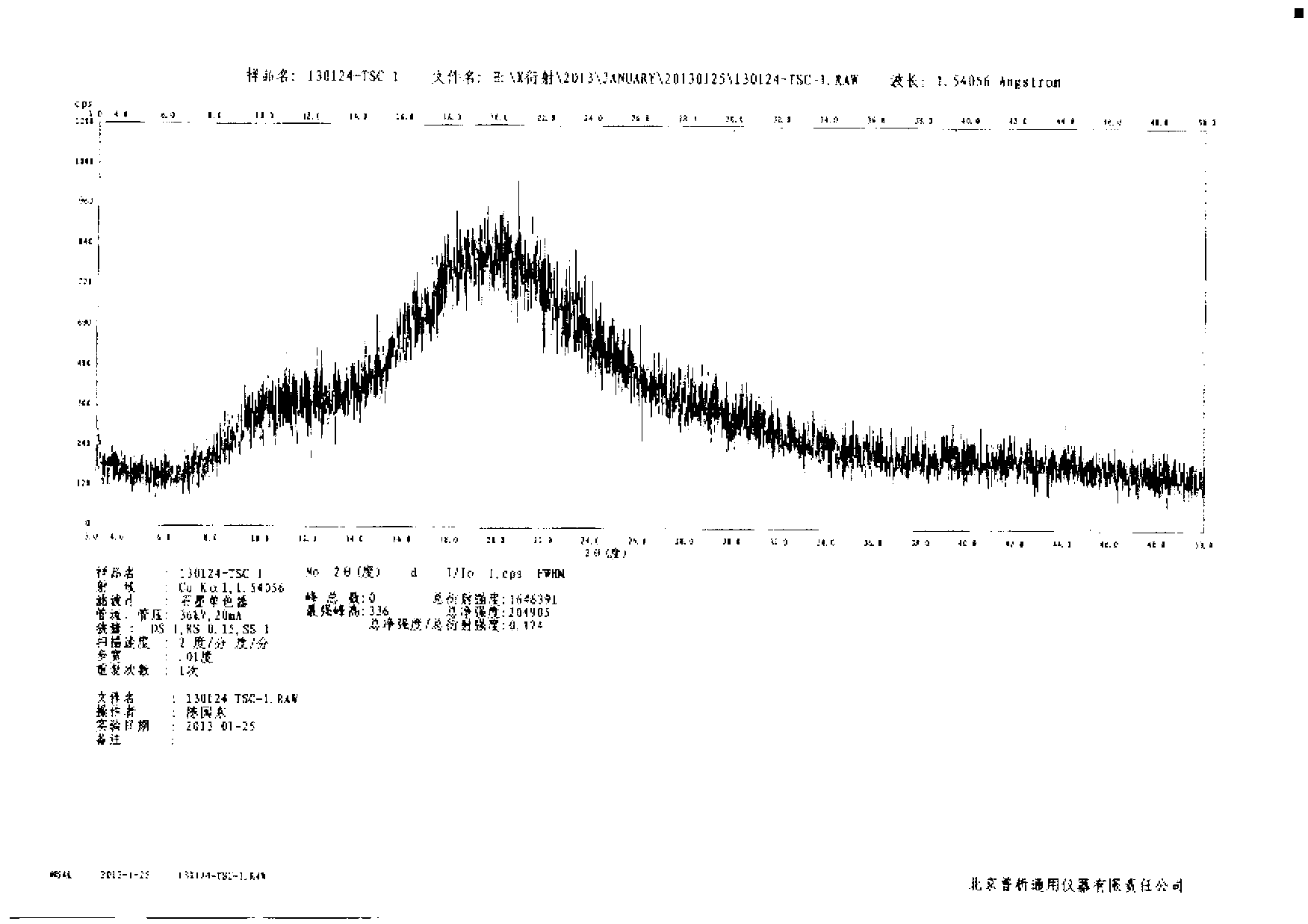
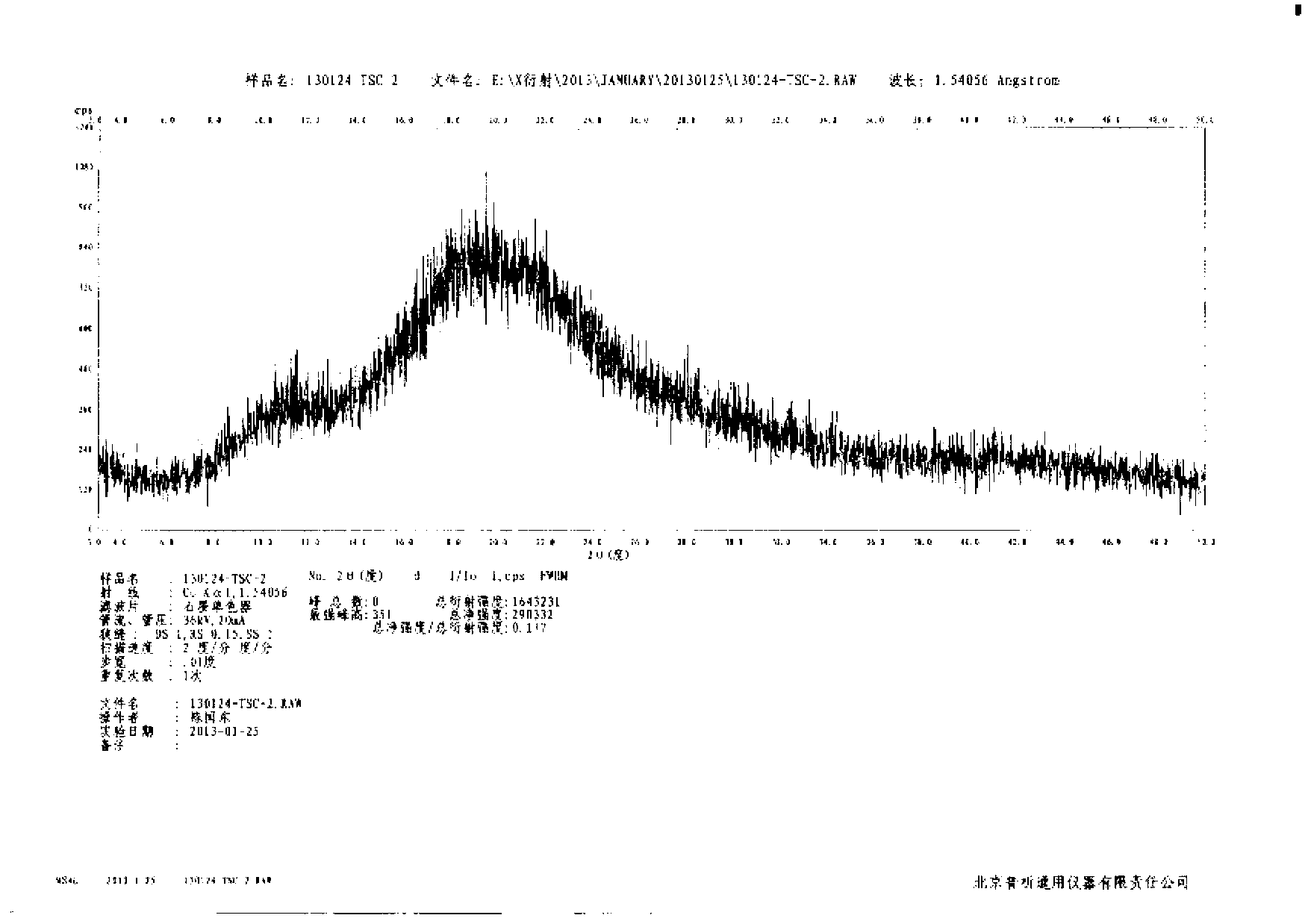
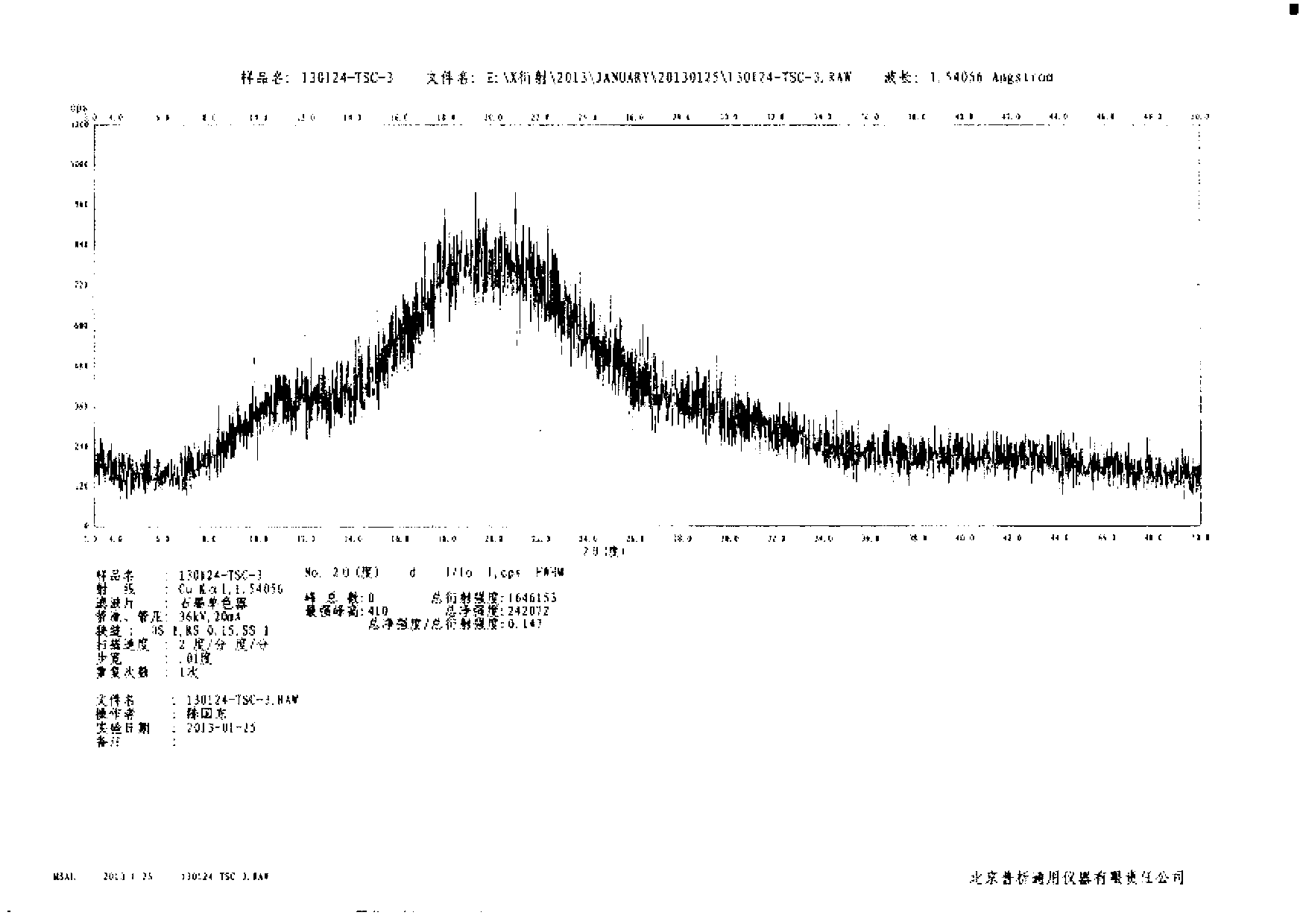
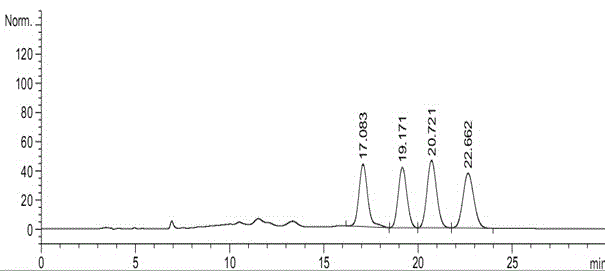
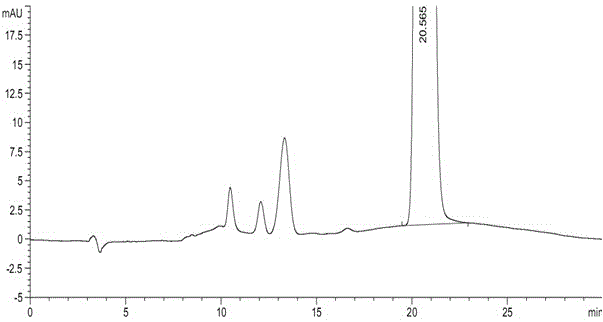
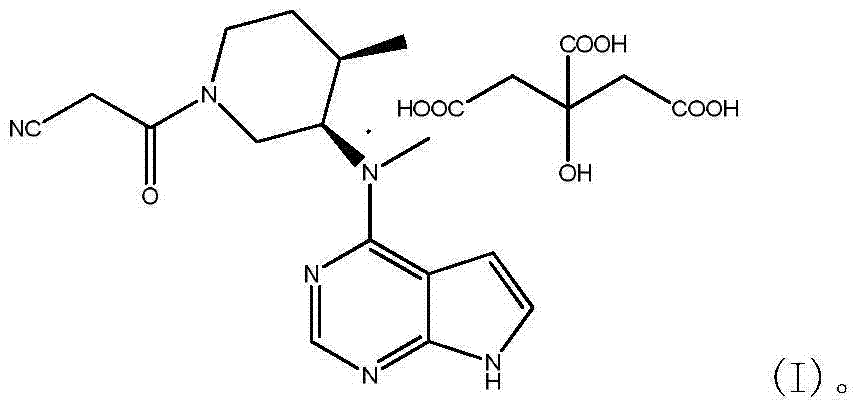
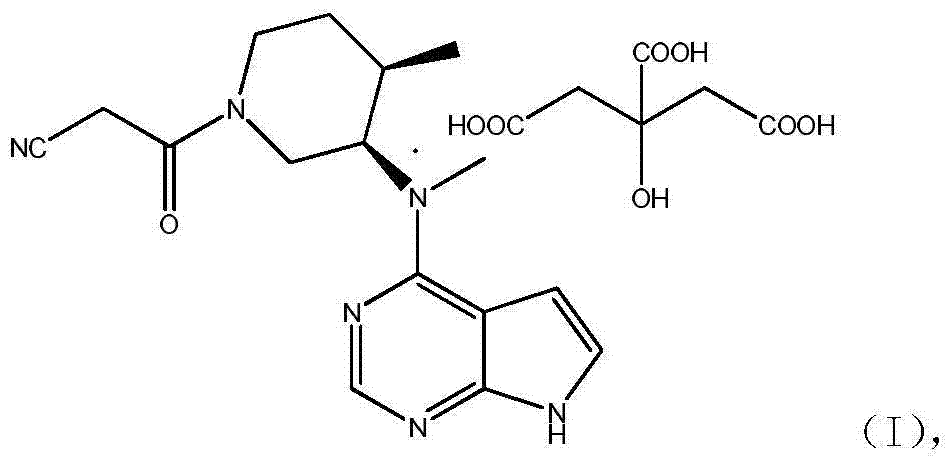
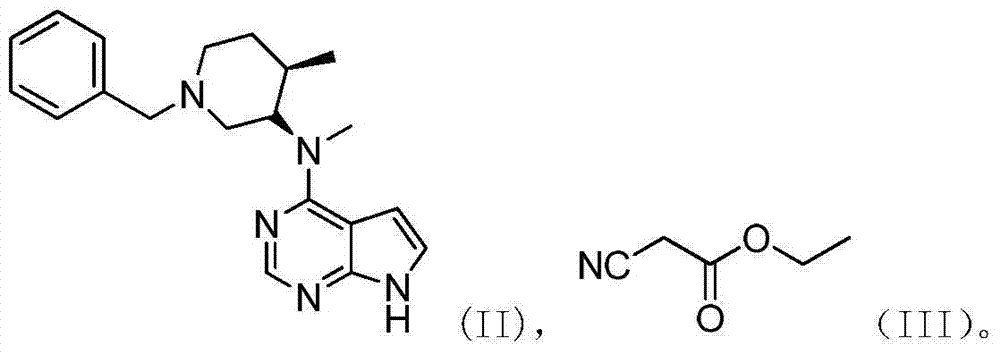
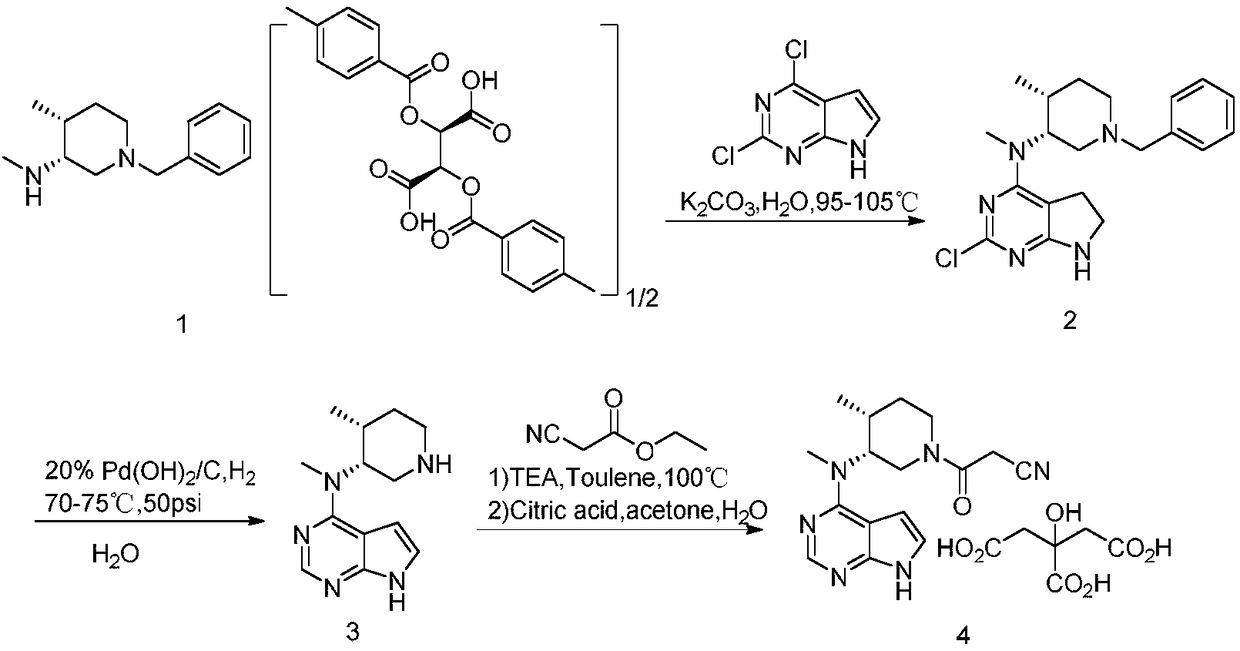
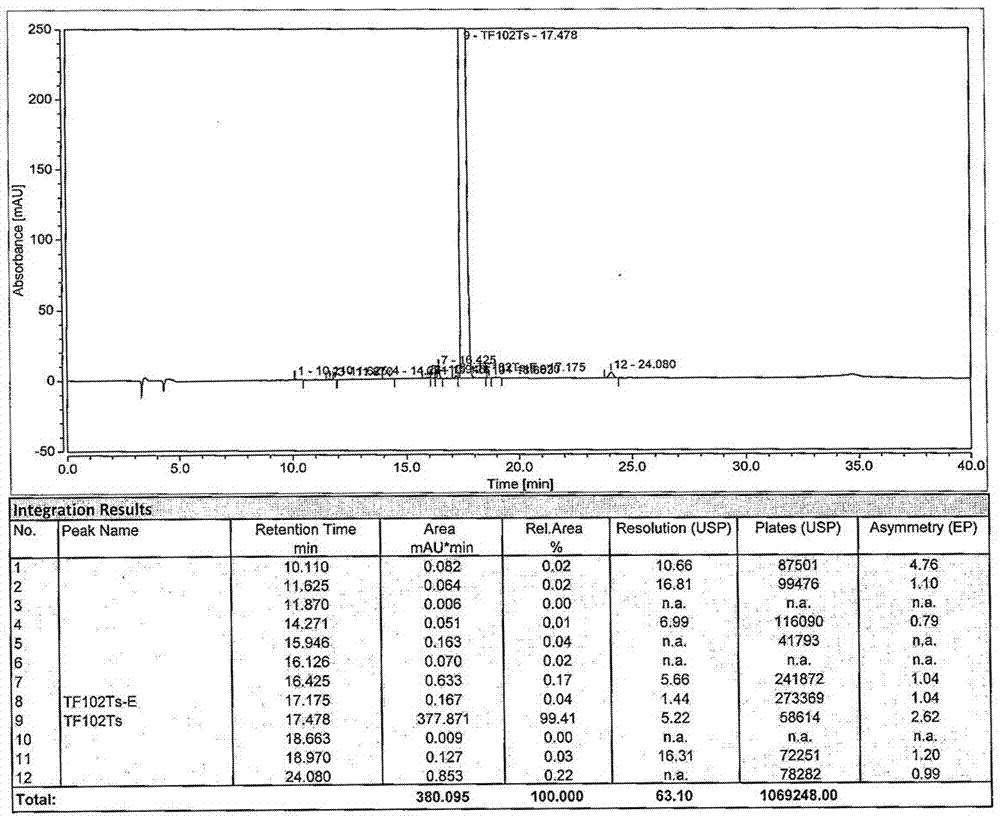
N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal
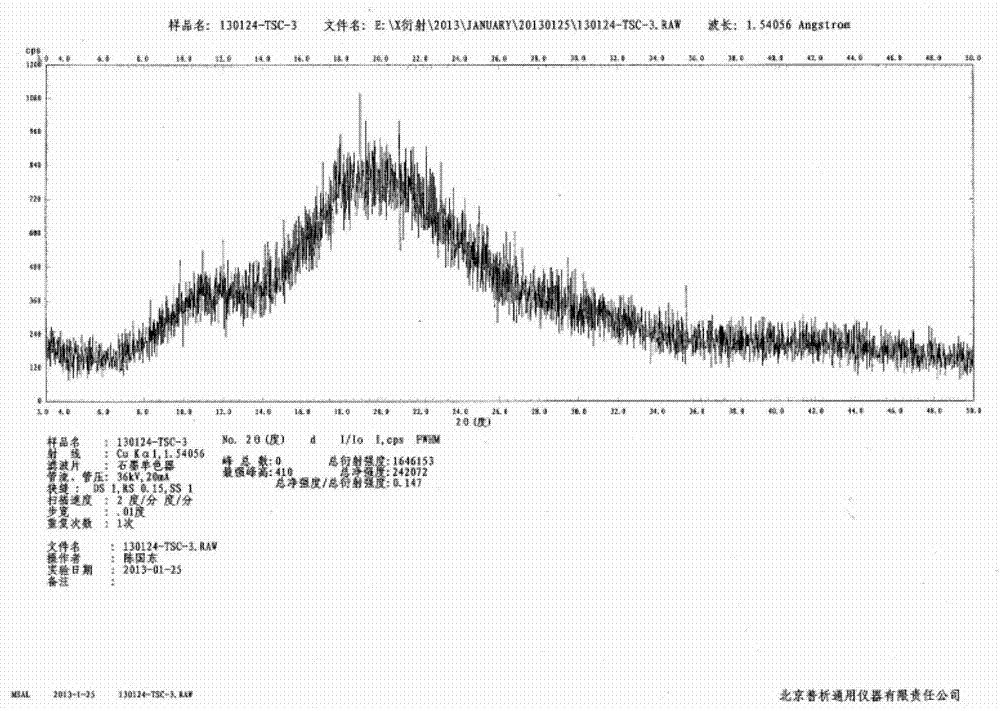
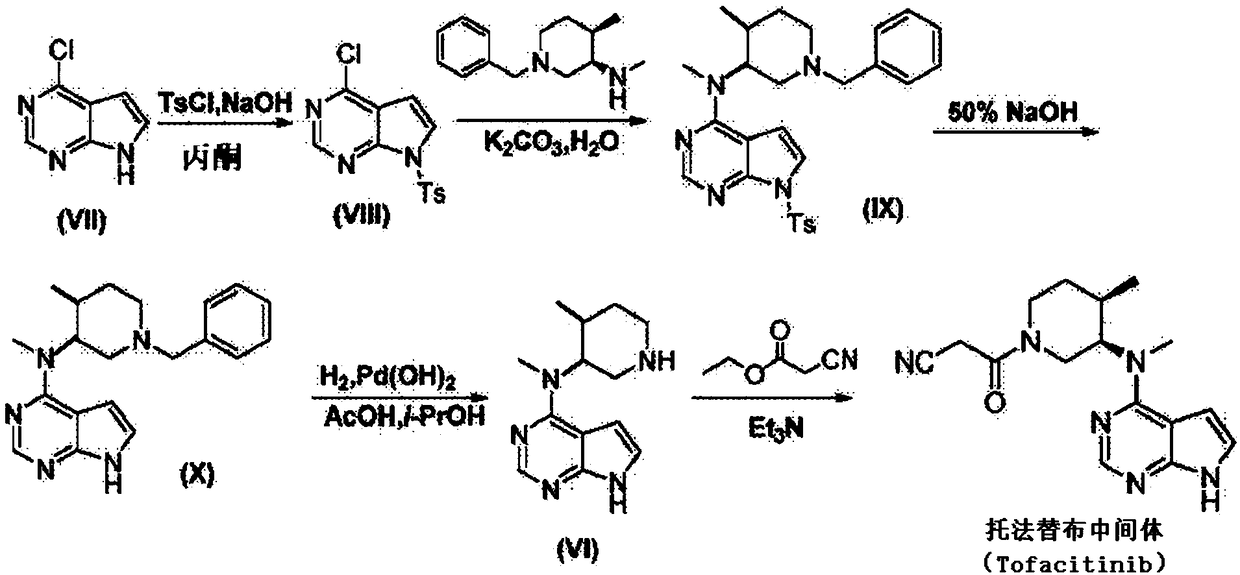
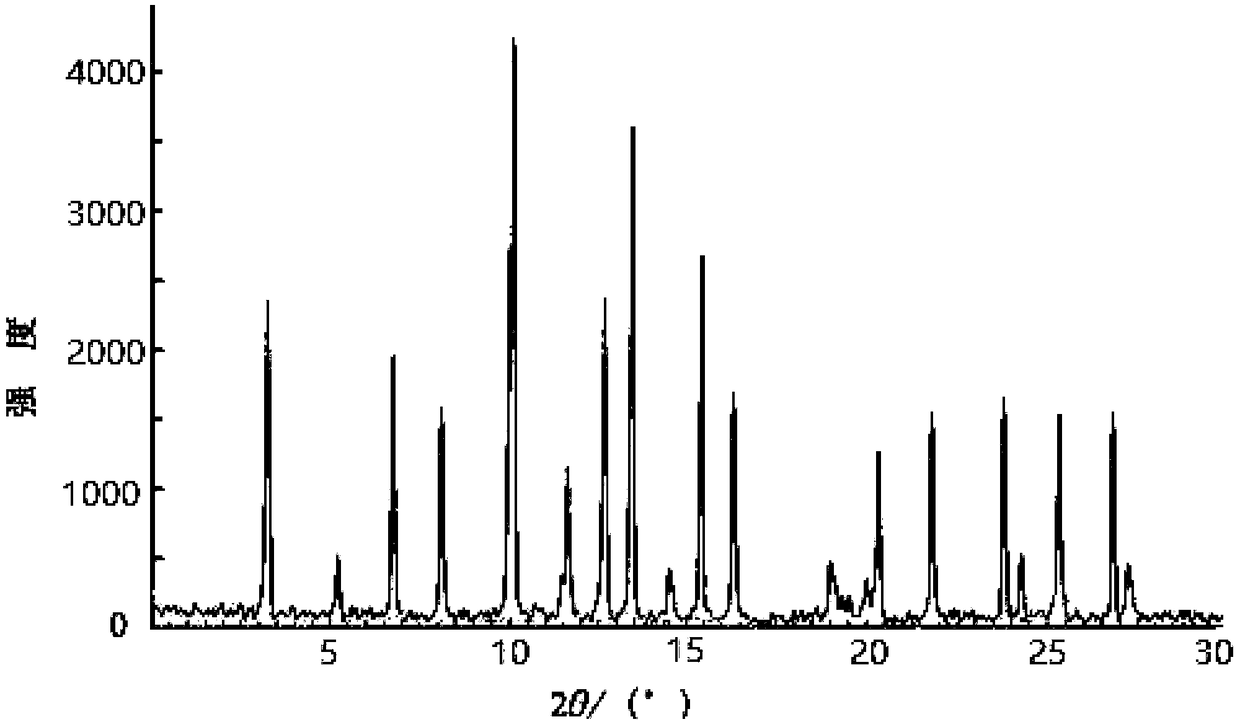
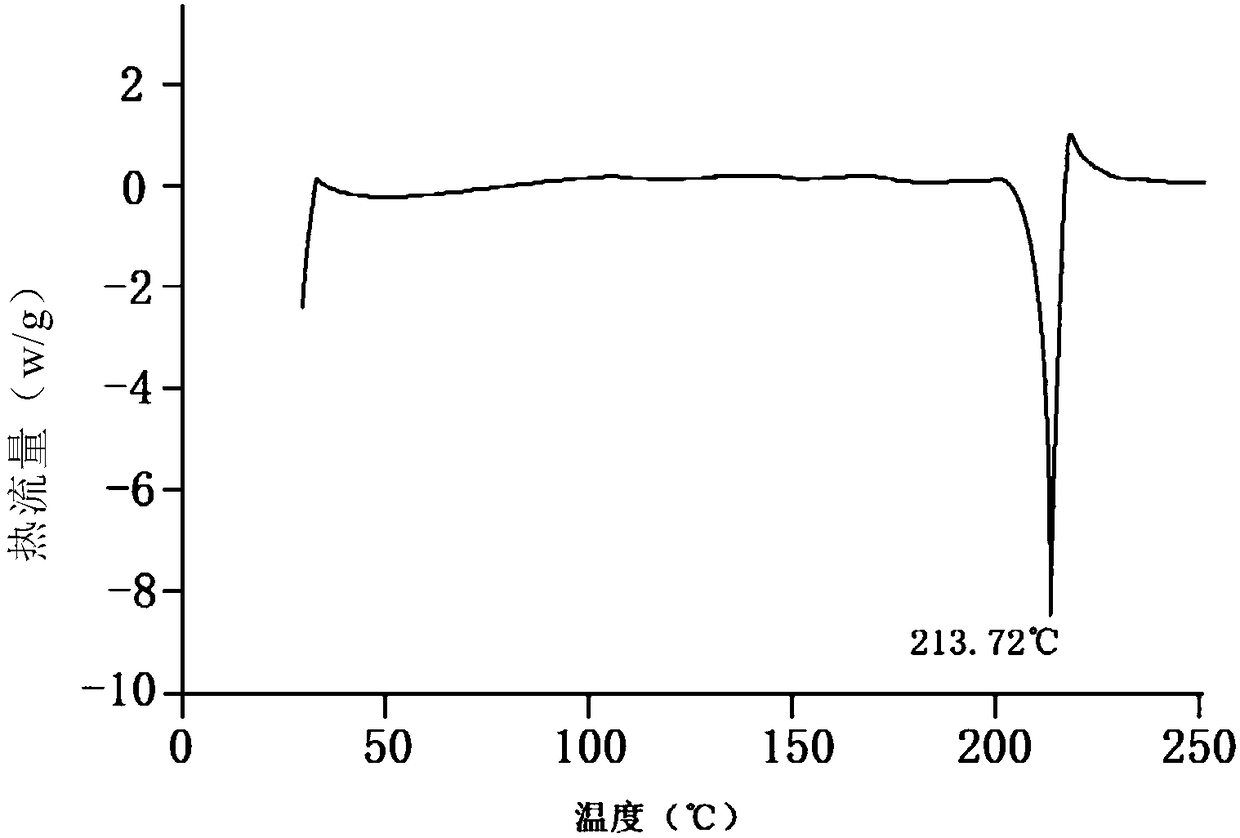
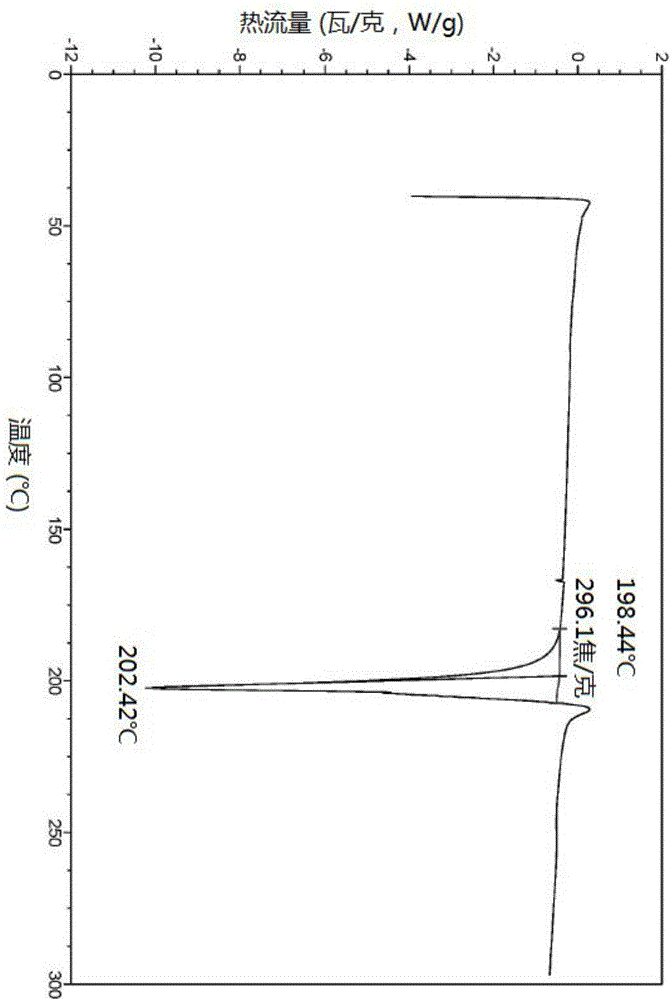
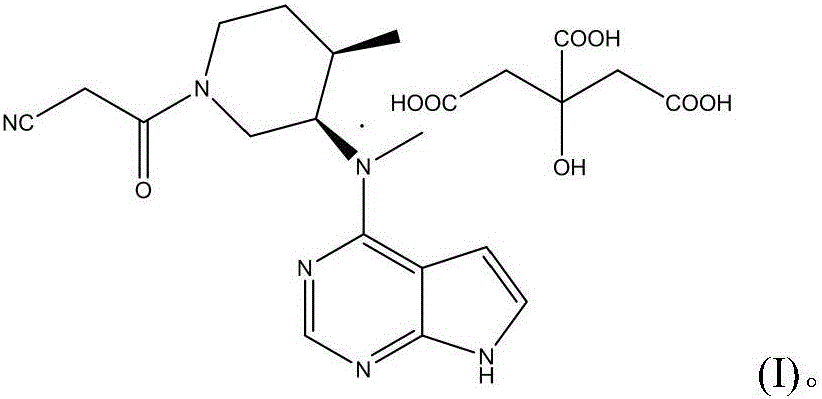
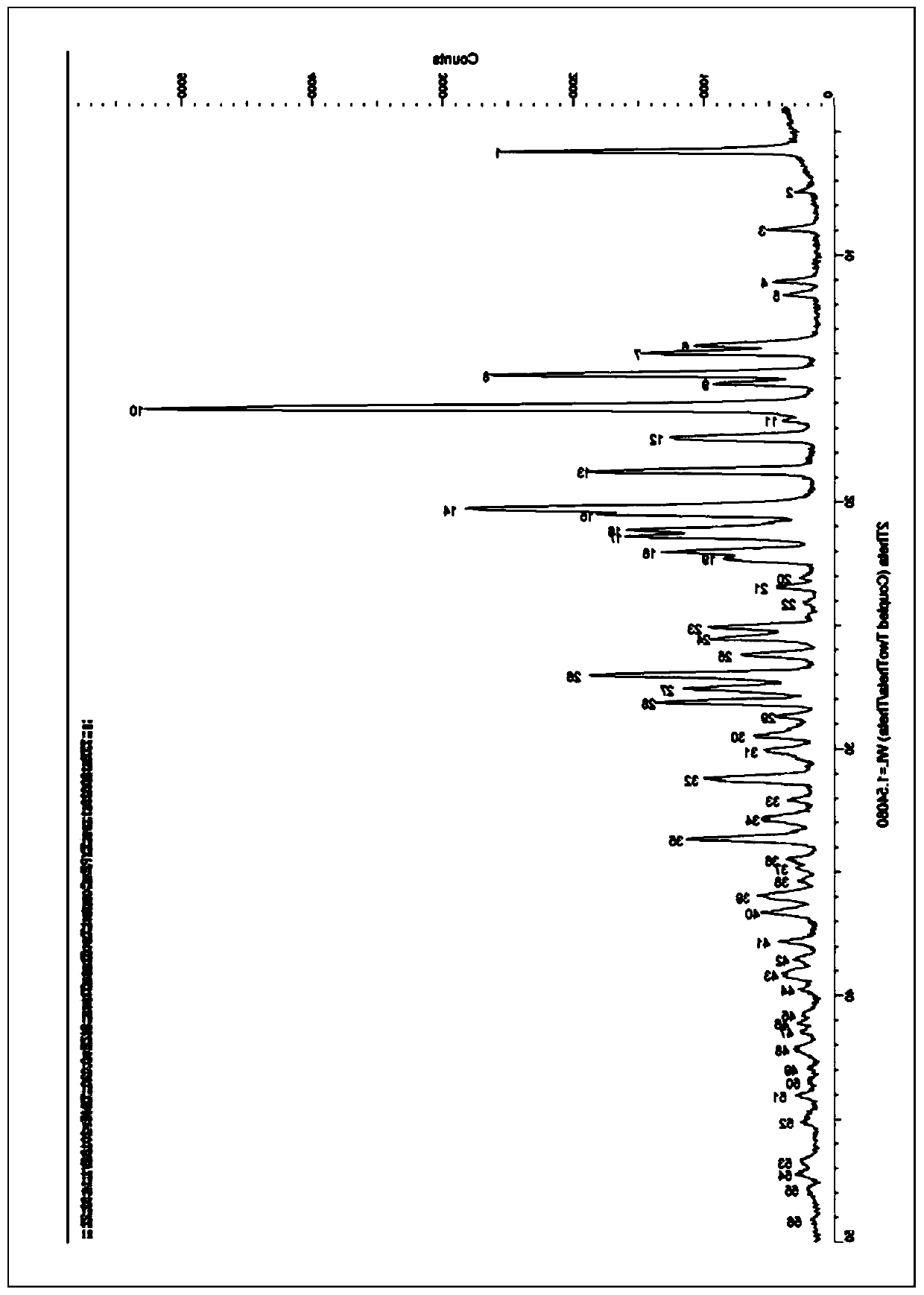
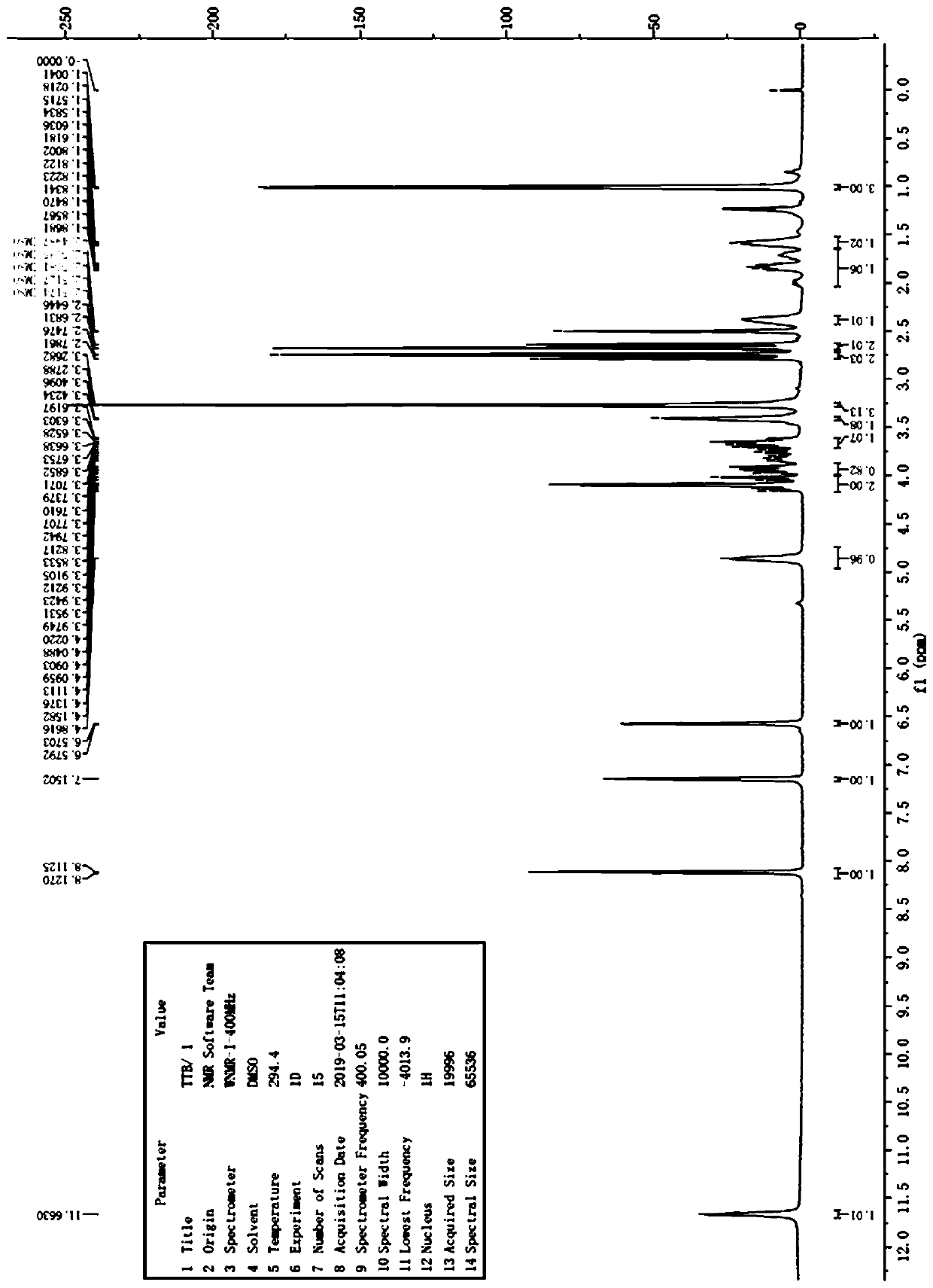
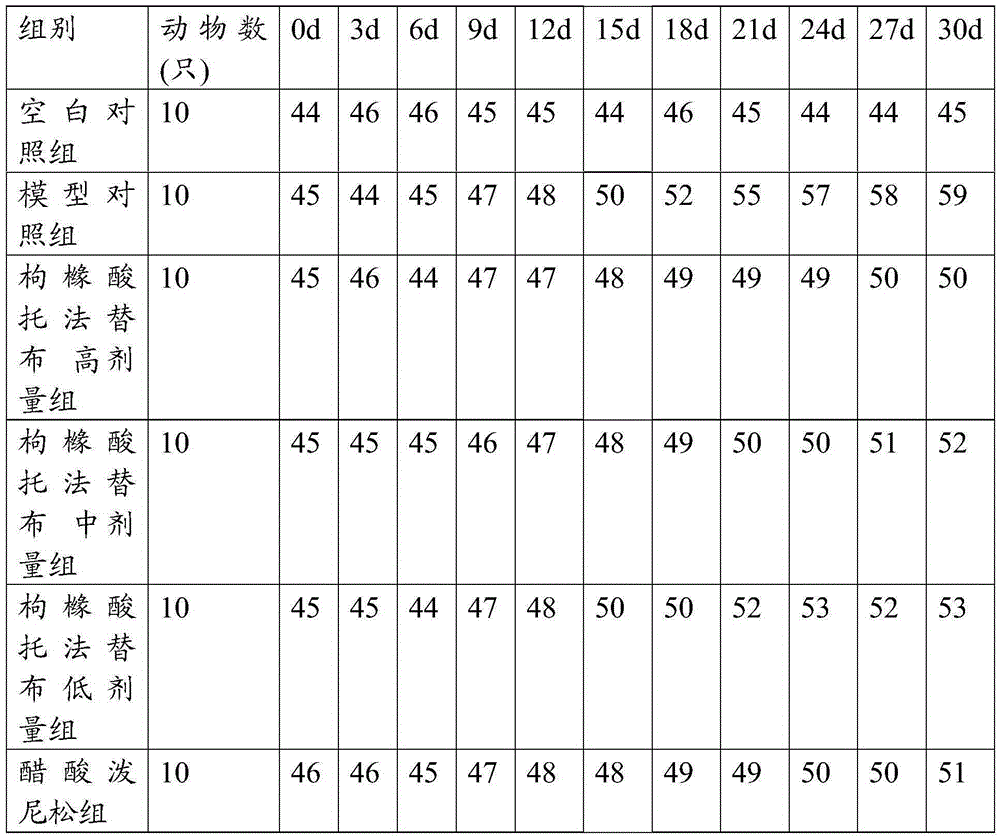
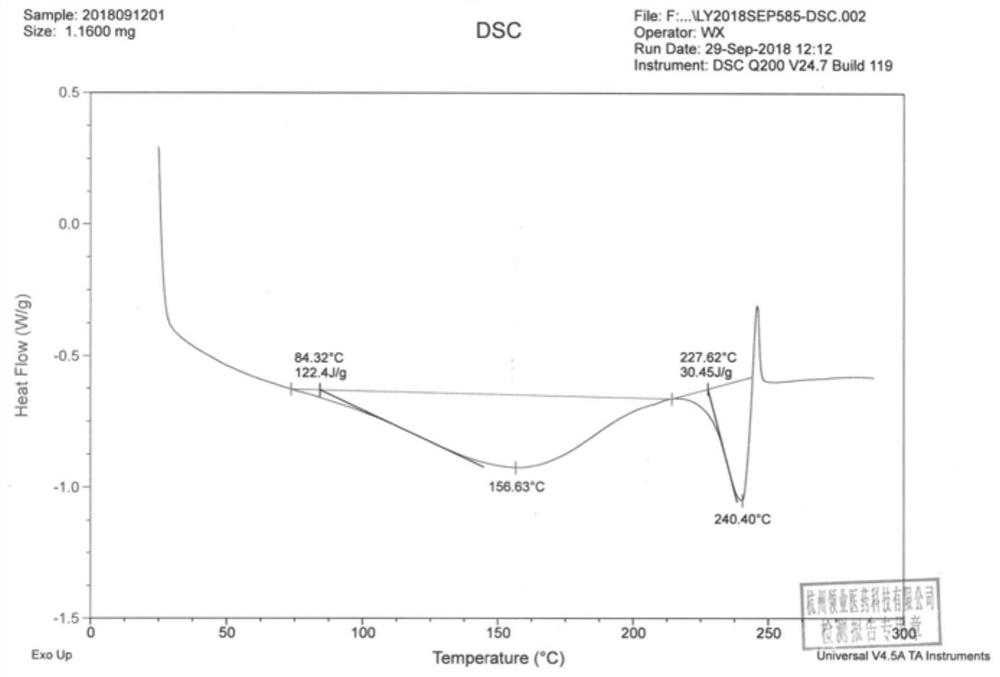
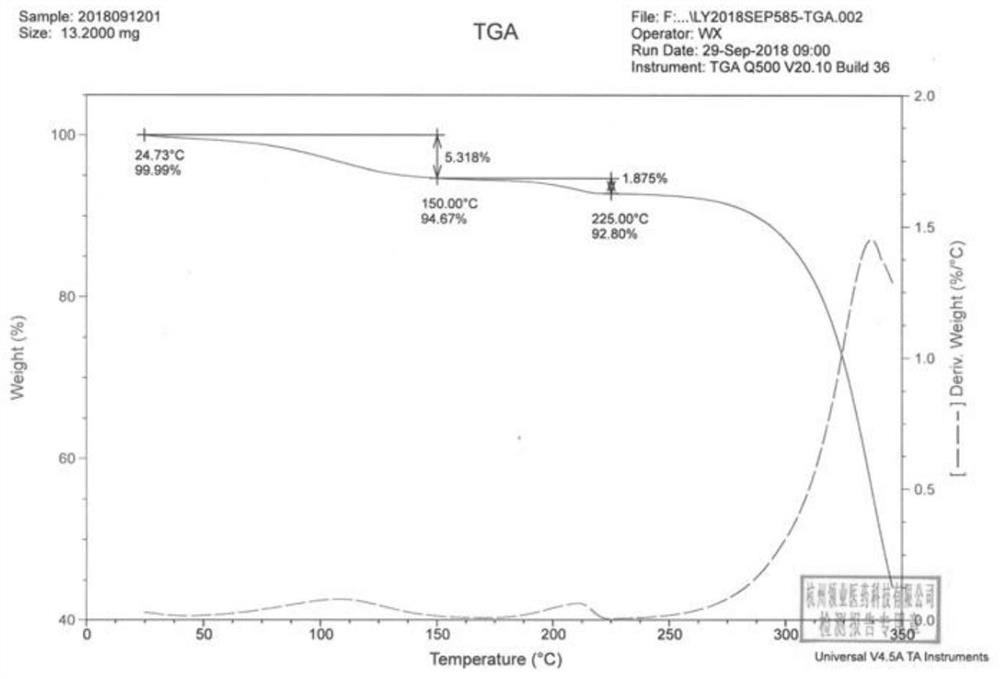
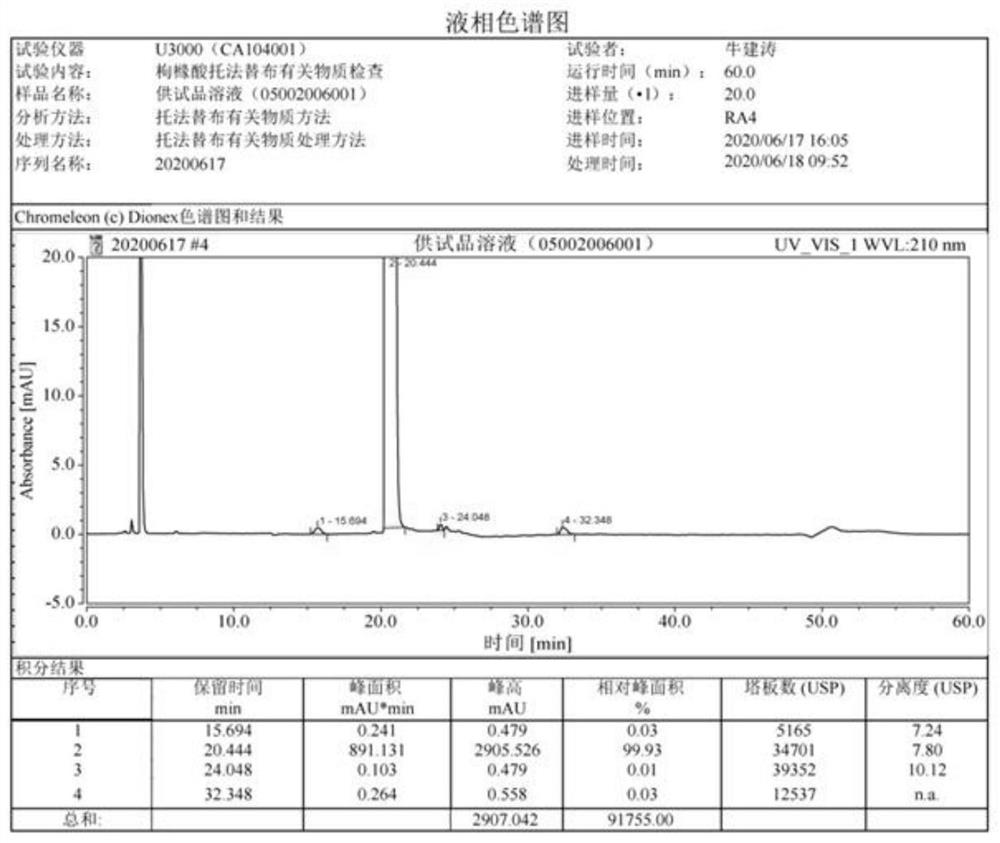
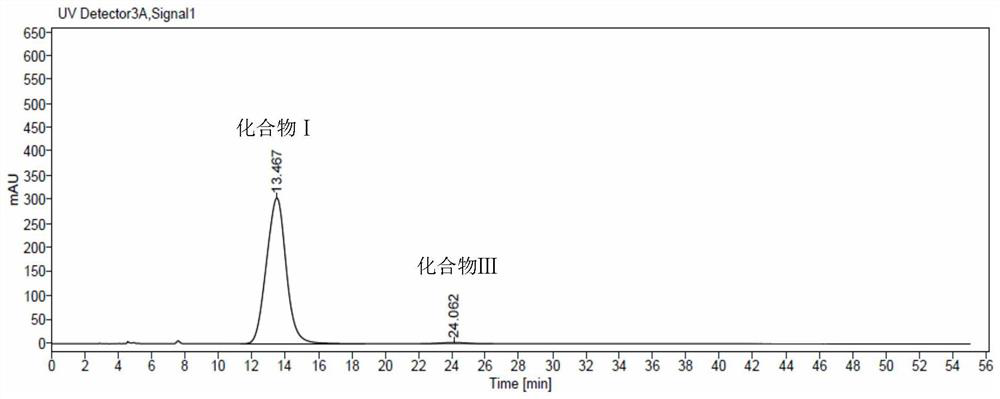
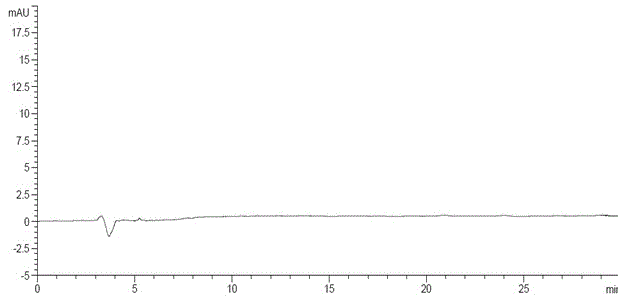
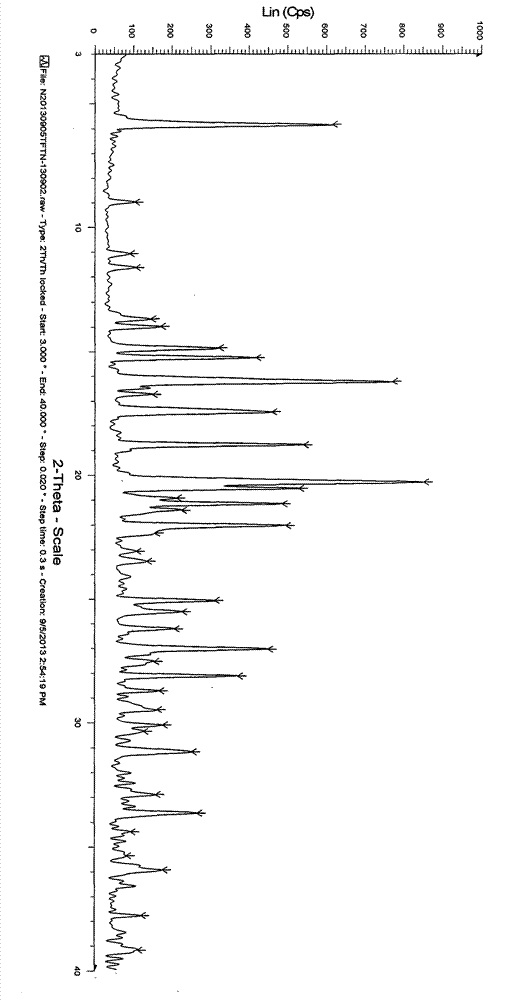
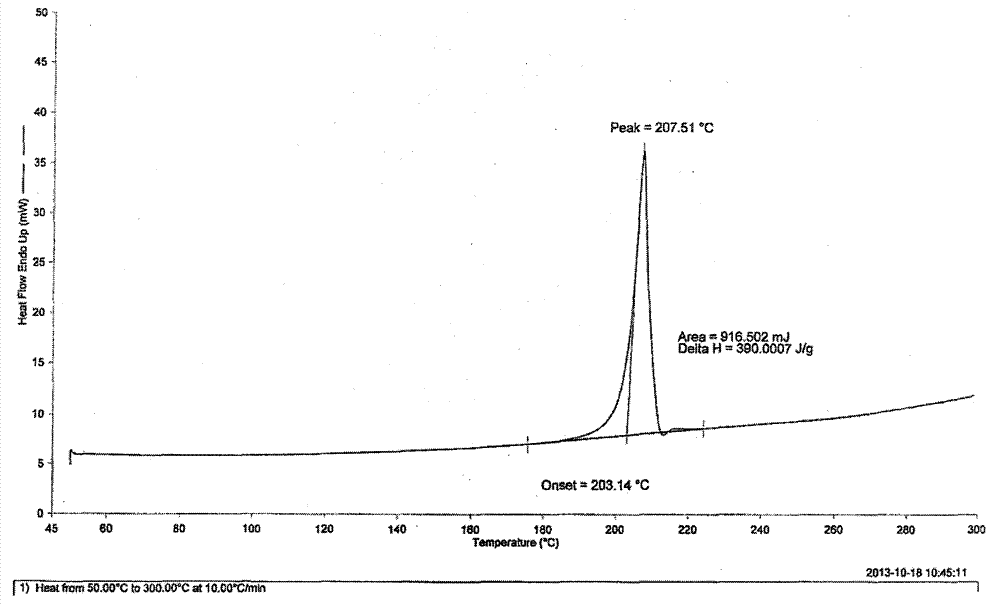
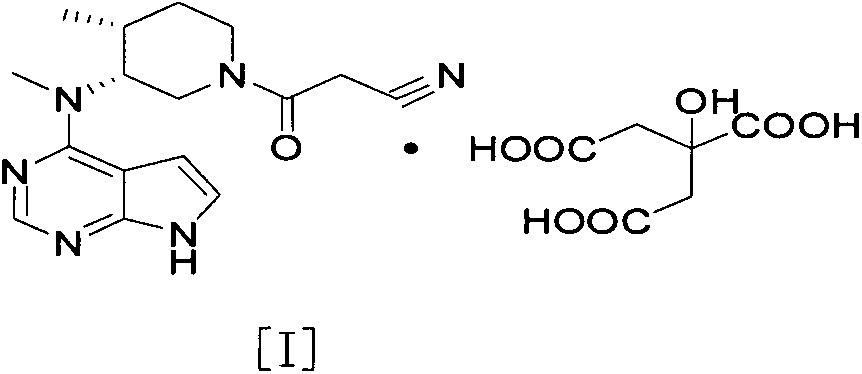
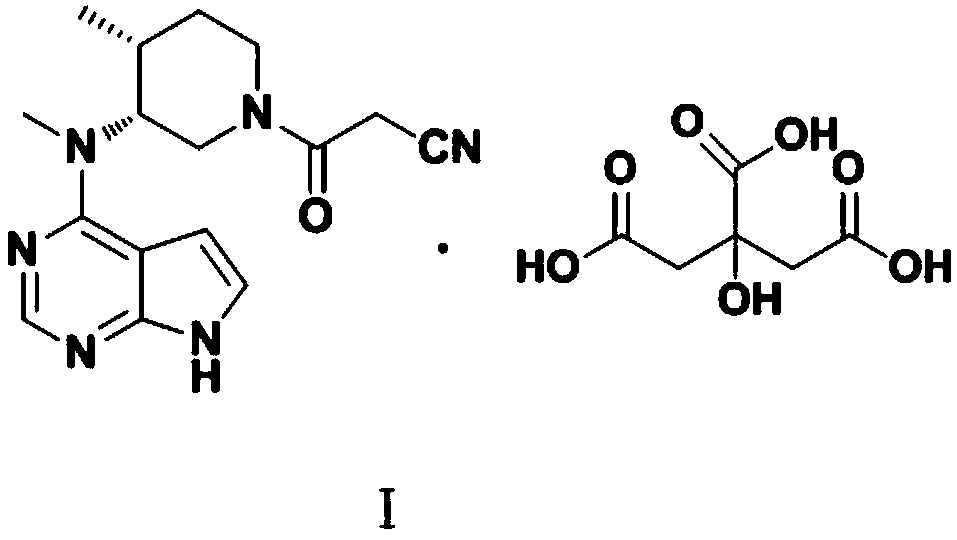
The invention discloses an N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal and a preparation method thereof. The method comprises steps as follows: firstly, 4-chloro-7-(p-tolylsulfonyl) pyrrolo [2,3-d] pyrimidine, 1-benzyl-4-methyl-3-aminomethyl dihydrochloride, potassium carbonate and water are added to a reaction vessel sequentially, heated and subjected to thermal insulation, and white solids are obtained; the white solids, sodium hydroxide and n-butyl alcohol are added to the reaction vessel sequentially, an obtained organic phase is washed with water, then anhydrous magnesium sulfate is added to the organic phase for dehydration, and a dichloromethane extracting solution is obtained; finally, eluting and curing are performed, and the crystal is obtained. The purity of the crystal is higher than 99.9%. According to a final product tofacitinib citrate synthesized with the intermediate, related substances are controlled to be lower than 0.1%, and the preparation of the crystal has great technical advantage and research value.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
Tofacitinib citrate sustained release tablet and preparation method thereof
InactiveCN111184696AOrganic active ingredientsAntipyreticProlonged-release tabletCombinatorial chemistry
The invention belongs to the field of pharmaceutical preparations, and specifically relates to a tofacitinib citrate sustained release tablet and a preparation method thereof. The sustained release tablet includes tofacitinib citrate, a skeleton material and a filling agent; and the skeleton material includes a corrosive skeleton material and a hydrophilic gel skeleton material. The sustained release tablet is simple in preparation technology, high in process reproducibility and suitable for large-scale industrial production.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
A kind of preparation method of amorphous tofacitinib citrate
InactiveCN103073552BImprove solubilityImprove stabilityOrganic chemistryCITRATE ESTEROrganic solvent
The invention provides a preparation method for amorphous tofacitinib citrate. The preparation method is simple, can easily obtain high-purity amorphous tofacitinib citrate, and is suitable for industrial application. The preparation method includes the following steps: under the temperature range between 30 DEG C and 50 DEG C, organic solvent is used for dissolving tofacitinib citrate, so that solution is produced, water which is 15 DEG C to 25 DEG C is added into the solution, so that precipitate is produced, the precipitate is put in the environment of 15 DEG C to 25 DEG C for 4 to 24 hours, and the amorphous tofacitinib citrate is then recovered.
Owner:BEIJING PHARMA GRP CO LTD
Novel preparation method of tofacitinib citrate
PendingCN108484607AHigh purityNo reduction in solubilityOrganic active ingredientsOrganic chemistry methodsSolventCitric Acid Monohydrate
The invention discloses a novel preparation method of tofacitinib citrate, and particularly provides a preparation method of tofacitinib citrate. The preparation method comprises the following steps:adding a tofacitinib intermediate crude product in a solvent, completely dissolving at a relatively high temperature, and adding medicinal activated carbon and activated aluminum oxide for stirring and adsorption; filtering to remove the medicinal activated carbon and the activated aluminum oxide, adding a pre-cooled alkaline aqueous solution in a filtrate, reducing the temperature to 0-5 DEG C for crystallization, washing and then drying to obtain a tofacitinib intermediate refined product; dissolving citric acid monohydrate in an ethanol aqueous solution, then adding the tofacitinib intermediate refined product and crystallizing to obtain the tofacitinib citrate. The novel preparation method disclosed by the invention has the benefits that hard-to-remove pigments and impurities in the tofacitinib intermediate crude product can be significantly reduced, and further, the high-purity tofacitinib intermediate refined product is obtained.
Owner:科兴生物制药股份有限公司
Tofacitinib-citrate tablet and method for preparing tofacitinib-citrate tablet
InactiveCN106420648AOvercome sticking problemsEasy to operateOrganic active ingredientsAntipyreticMedicineLactose
The invention relates to a tofacitinib-citrate tablet and a method for preparing the tofacitinib-citrate tablet, and belongs to the technical field of medical preparations. The tofacitinib-citrate tablet provided by the invention is prepared from an active ingredient, an auxiliary material and coating powder, wherein the active ingredient is tofacitinib citrate; the auxiliary material is prepared from the following raw materials in percentage by weight: 57 to 62 percent of microcrystalline cellulose, 30 to 35 percent of lactose, 2.0 to 3.0 percent of croscarmellose sodium, 3.0 to 4.0 percent of compound lubricant and 0.5 to 1.5 percent of glidant; metering is carried out according to that the total mass of the active ingredient and the auxiliary material is 100 percent. According to the tofacitinib-citrate tablet provided by the invention, the raw material formula is changed; the sticking problem is solved. The invention provides a method for preparing the tofacitinib-citrate tablet, which is simple to operate and good in reproducibility, at the same time.
Owner:山东淄博新达制药有限公司
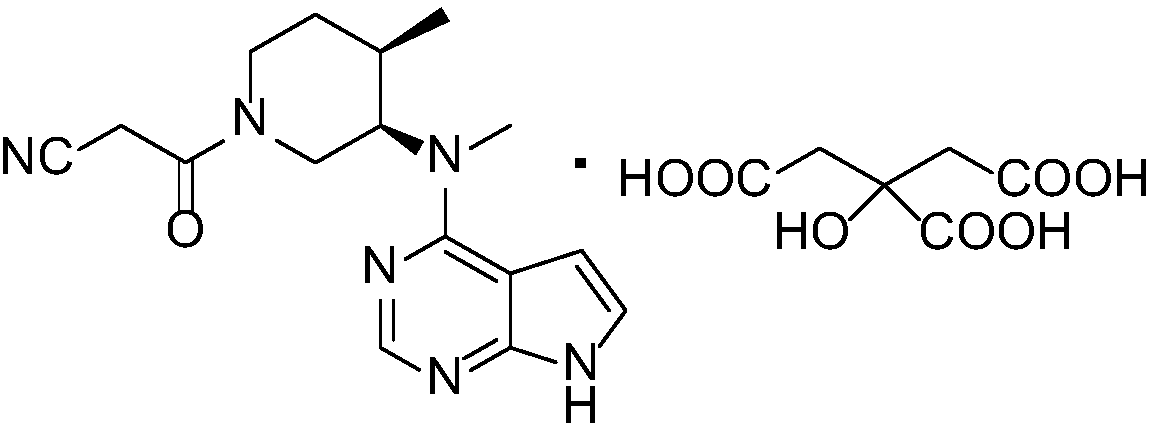
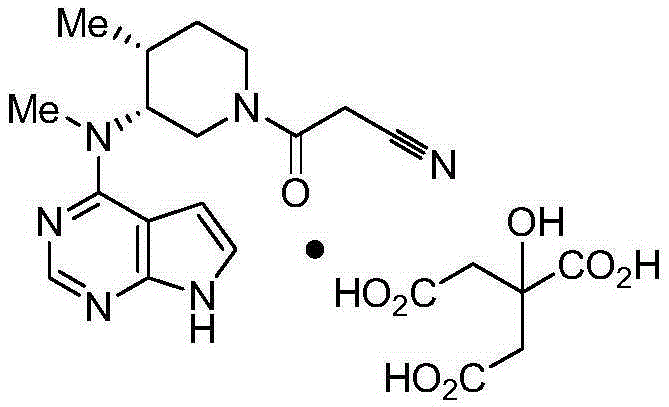
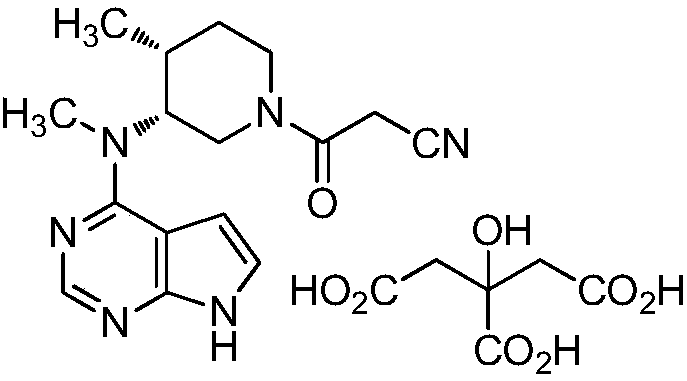
Tofacitinib citrate
ActiveCN105873931AImprove liquidityGood compressibilityOrganic active ingredientsNervous disorderCrystalline particleTOFACITINIB CITRATE
Crystalline particle of tofacitinib citrate, process for the preparation of a crystalline particle of tofacitinib citrate, pharmaceutical composition, substantially pure amorphous form of tofacitinib citrate, process for preparing the substantially pure amorphous form of tofacitinib citrate, and use of the crystalline particle of tofacitinib citrate, and the pharmaceutical composition are provided.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method for tofacitinib citrate
The invention relates to the field of medicinal chemistry, and provides a preparation method for tofacitinib. ((3R,4R)-1-benzyl-4-methyl-piperidine-3-radical)-methyl-(7H-pyrrolo [2, 3-D] pyrimidine-4-radical)-amine is taken as a starting material, after the hydrogenation debenzylation reaction and amidation, tofacitinib is prepared, and after the tofacitinib and citric acid are salified, the tofacitinib citrate is obtained. According to the developed technology, the product quality is quite high, the purity is 99.9% or above, the content of any individual impurity is less than 0.1%, and the quality standard of tofacitinib citrate medicine as a registered active pharmaceutical ingredient is met. The hydrogenation reaction utilizes an aqueous hydrochloric acid solution as a solvent, and thehydrogenation reaction is quite safe, green and environmentally friendly.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Preparation method of tofacitinib citrate
ActiveCN108276414AThorough responseHigh purityOrganic chemistry methodsCarboxylic acid salt preparationSolventEthyl fumarate
The invention discloses a preparation method of tofacitinib citrate. The preparation method comprises the following steps: stirring an organic solvent, a compound 3, 5 percent wet palladium on carbonand acetic acid until a mixture is suspended; conveying the mixture into a micro-reactor from a channel A; meanwhile, conveying alkylsilane into the micro-reactor from a channel B; controlling the temperature of the micro-reactor to 20 to 30 DEG C and carrying out reaction; carrying out post-treatment to obtain a compound 2; after uniformly mixing an alcohol type solvent, the compound 2, ethyl cyanoacetate and DBU (1,8-Diazabicyclo [5,4,0]undec-7-ene), conveying a mixture into the micro-reactor from the channel A; dissolving citric acid into a mixed solution of water and the alcohol type solvent, conveying a mixture into the micro-reactor from the channel B; controlling the temperature in the micro-reactor to 70 to 80 DEG C and carrying out reaction; cooling and crystallizing to obtain thetofacitinib citrate. The method provided by the invention has the advantages of controllable reaction condition and simplicity in operation; dangers caused by the fact that flammable and explosive gas is used are avoided and the safety is higher; reaction can be finished instantly and the reaction time is greatly shortened; debenzylation reaction and acylation reaction are complete and post-treatment is simple; the preparation method is suitable for industrial large-scale production. A formula is shown in the description.
Owner:山东安信制药有限公司
Efficient method for the preparation of tofacitinib citrate
ActiveUS20160297825A1Organic compound preparationOrganic chemistry methodsCombinatorial chemistryIndustrial scale
Disclosed is a novel process for the synthesis of tofacitinib citrate on an industrial scale with high yields and purity starting with cis-(1-benzyl-4-methyl-piperidin-3-yl)methylamine bis-hydrochloride racemate (intermediate VIII), which comprises:1. Condensation between intermediates VII and VIII to give intermediate VI2. Hydrogenation of intermediate VI to give intermediate V3. Resolution of intermediate V to give intermediate IV with enantiomeric purity >99%4. Release of intermediate IV in a basic medium to give intermediate III5. N-acylation reaction of intermediate III to give II (tofacitinib)6. Salification of intermediate II to give tofacitinib monocitrate (I)
Owner:OLON
Method for preparing Tofacitinib citrate
The invention provides a method for preparing Tofacitinib citrate. The method comprises the steps of hydrochloric acid removal, amidation reaction, salt forming and the like. According to the method,the cost is low, the operation is simple and convenient, and the obtained product is high in purity, so that the method is applicable to industrial large-scale production.
Owner:科兴生物制药股份有限公司
Tofacitinib citrate composition and preparation method thereof
The invention relates to a tofacitinib citrate oral solution, in particular to a tofacitinib citrate oral solution with more stable pharmaceutical properties and a preparation method of the tofacitinib citrate oral solution. The tofacitinib citrate oral solution is prepared from tofacitinib citrate, a pH regulator, a preservative, a flavoring agent and water. The tofacitinib citrate oral solutionhas better stability and is suitable for children and old people with dysphagia.
Owner:南京安美医药科技有限公司
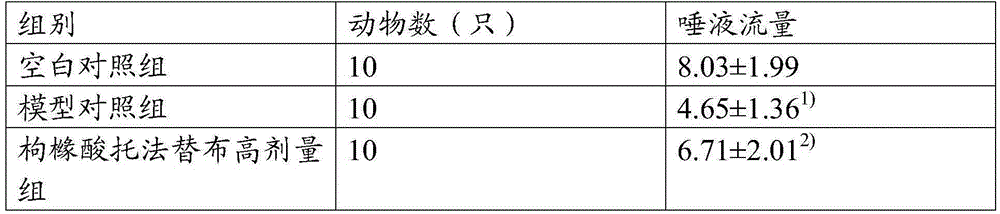
Application of tofacitinib citrate and medicinal composition thereof in preparation of medicament for treating sjogren's syndrome
InactiveCN105326844AImprove the symptoms of dry mouth and eyesAchieve therapeutic effectOrganic active ingredientsImmunological disordersMedicineSicca syndrome
The invention discloses application of tofacitinib citrate and medicinal composition thereof in preparation of a medicament for treating sjogren's syndrome. The medicinal composition contains the tofacitinib citrate and / or optimal pharmaceutically-acceptable carriers or excipient. According to the medicinal composition, the sjogren's syndrome of mouths and eyes is obviously improved.
Owner:NINGBO LIWAH PHARM CO LTD
Tofacitinib citrate intermediate as well as preparation method and application thereof
ActiveCN111995627AGood effectReduce manufacturing costOrganic compound preparationCarboxylic acid salt preparationPharmaceutical SubstancesPyrrole
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a tofacitinib citrate intermediate as well as a preparation method and an application thereof. Whereinthe tofacitinib citrate intermediate is N-methyl-N-((3R, 4R)-4-methylpiperidine-3-yl)-7H-pyrrolo [2, 3-D] pyrimidine-4-amine dihydrochloride monohydrate. The preparation method comprises the followingsteps: adding N-methyl-N-((3R, 4R)-1-benzyl-4-methylpiperidine-3-yl)-N-methyl-7H-pyrrolo [2, 3-d] pyrimidine-4-amine into water and an organic solvent, then adding hydrochloric acid and palladium hydroxide carbon, introducing hydrogen to react, and filtering out the palladium hydroxide carbon; cooling to room temperature, dropwise adding an organic solvent, crystallizing, carrying out suction filtration, and drying to obtain the tofacitinib citrate intermediate. The method greatly improves the utilization rate of raw materials, reduces the production cost, and improves the product quality.
Owner:山东金城昆仑药业有限公司
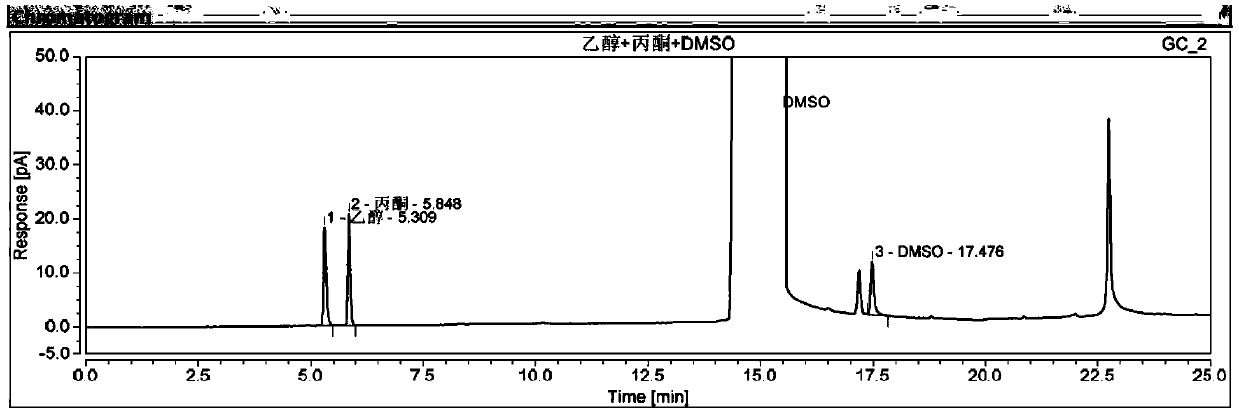
Tofacitinib Citrate residual solvent GC detection method
InactiveCN110286160AEfficient determinationAchieve separationComponent separationGas phaseAnalytical technique
The invention relates to the technical field of drug analysis, in particular to a gas-liquid chromatography detection method of a tofacitinib citrate residual solvent. The method comprises the following steps of: configuring an analysis solution; setting chromatographic conditions; and performing determination on a machine. The method can effectively determine the content of the tofacitinib citrate residual solvent, thereby ensuring the controllable quality of the product.
Owner:NANJING GRITPHARMA CO LTD
Method for detecting tofacitinib chiral intermediates and enantiomer thereof
ActiveCN112697906AEfficient separationHigh sensitivityComponent separationCarbamateFluoroacetic acid
The invention belongs to the field of analytical chemistry, and particularly relates to a citric acid tofacitinib citrate chiral intermediate and an enantiomer thereof. A chromatographic column taking amylase tri(3,5-dimethyl phenyl carbamate) coated porous silica gel microspheres as a filler is adopted. A mixed solution of n-hexane-isopropanol-methanol-diethylamine- trifluoroacetic acid is used as a mobile phase for isocratic elution, and the solution enters a detector for detection. The citric acid tofacitinib citrate chiral intermediate and the enantiomer thereof can be effectively separated, and the method has the advantages of high sensitivity and separation degree, good repeatability and durability, simplicity in operation, and stable and reliable result. The method has extremely important significance in realizing the citric acid tofacitinib citrate chiral intermediate and tofacitinib citrate quality control.
Owner:SHANGHAI SCIENPHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8fe88b74-2a83-4028-9625-d9db2d7a0a7a/HDA0000777129110000011.PNG)
![N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8fe88b74-2a83-4028-9625-d9db2d7a0a7a/HDA0000777129110000012.PNG)
![N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d] pyrimidine-4-amine crystal](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8fe88b74-2a83-4028-9625-d9db2d7a0a7a/HDA0000777129110000021.PNG)