New method for preparing tofacitinib citrate crystal-form A
A technology of citric acid and new method, applied in the field of medicine, can solve the problems of low yield, poor reproducibility, unsuitable for industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
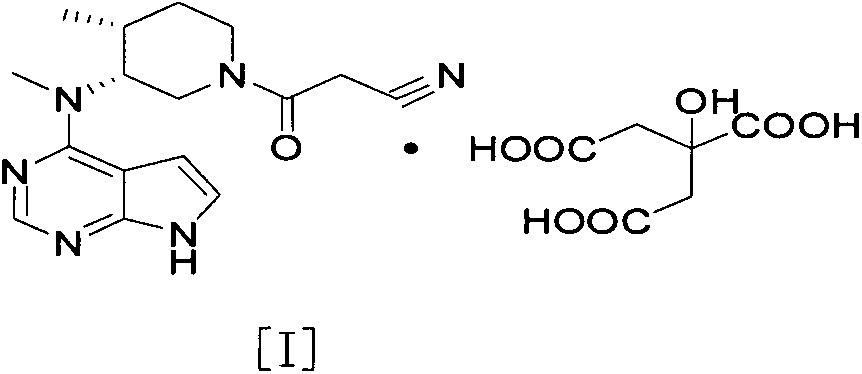
[0021] Example 1: 3-{4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3- Oxo-propionitrile monocitrate
[0022]
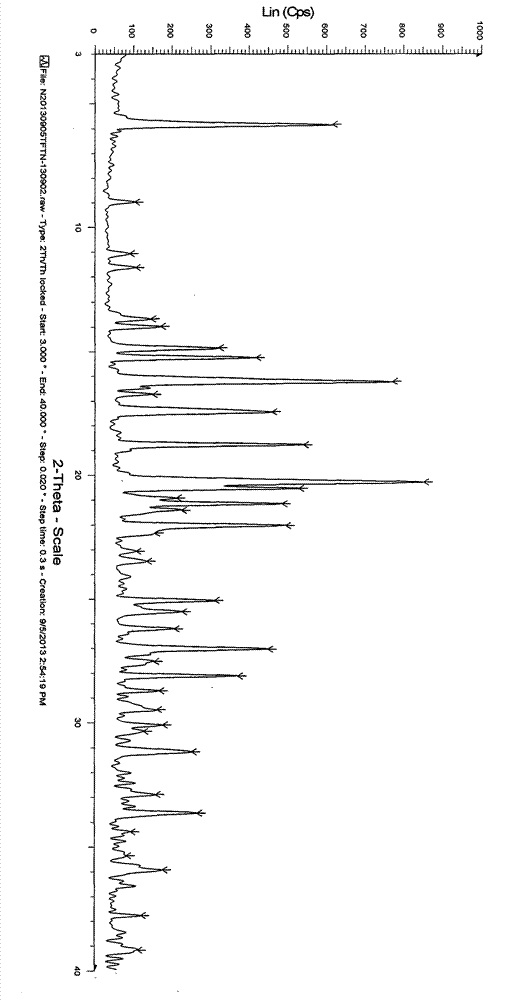
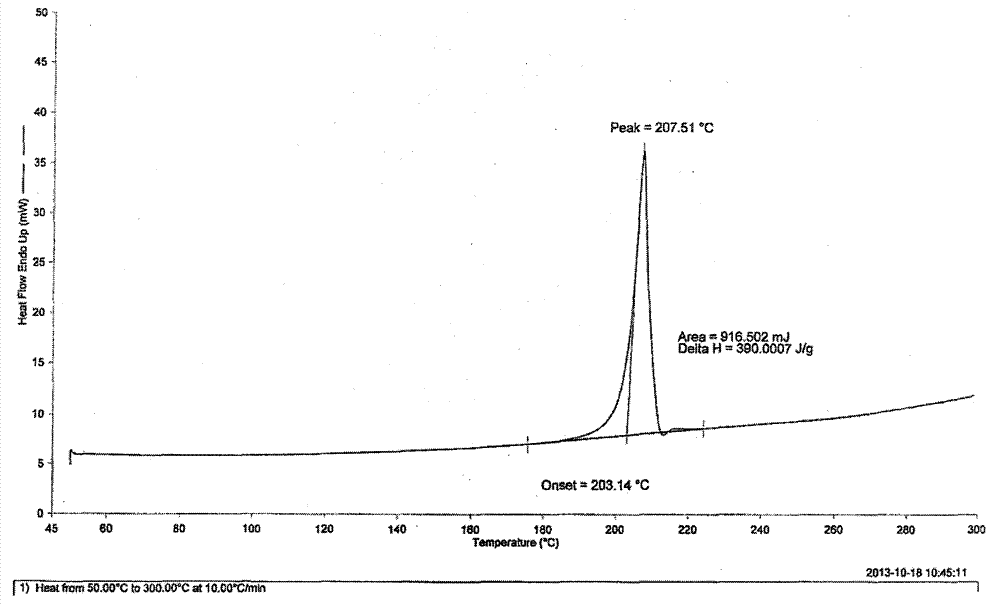
[0023] 3-{4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo- Propionitrile (5.0g / 16.0mmol) was dissolved in 75ml of acetone and heated to 60°C. To this solution was added dropwise a solution of citric acid (3.4 g / 16.2 mmol) in acetone (25 ml). The resulting mixture was cooled to room temperature and stirred for an additional 2 hours. The solid was collected by filtration, washed with acetone and dried under vacuum at 35°C to afford 5.6 g (69%) of a white crystalline powder. As determined by X-ray powder diffraction, it was shown that the generated crystal form was the target crystal form A.
Embodiment 2
[0024] Example 2: 3-{4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3- Oxo-propionitrile monocitrate
[0025] 3-{4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo- Propionitrile (5.0g / 16.0mmol) was dissolved in 25ml of tetrahydrofuran and heated to 60°C. To this solution was added dropwise a solution of citric acid (3.4 g / 16.2 mmol) in tetrahydrofuran (25 ml). The resulting mixture was cooled to room temperature and stirred for an additional 2 hours. The solid was collected by filtration, washed with tetrahydrofuran and dried under vacuum at 50°C to afford 4.3 g (53%) of a white crystalline powder. As determined by X-ray powder diffraction, it was shown that the generated crystal form was the target crystal form A.
Embodiment 3
[0026] Example 3: 3-{4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3- Oxo-propionitrile monocitrate
[0027] 3-{4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo- Propionitrile (5.0 g / 16.0 mmol) was dissolved in 40 ml of methanol and heated to 50°C. To this solution was added dropwise a solution of citric acid (3.4 g / 16.2 mmol) in methanol (25 ml). The resulting mixture was stirred at 50°C for 0.5 hours and then at room temperature for an additional 2 hours. The solid was collected by filtration, washed with methanol and dried under vacuum at 40°C to afford 6.8 g (84%) of a white crystalline powder. As determined by X-ray powder diffraction, it was shown that the generated crystal form was the target crystal form A.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com