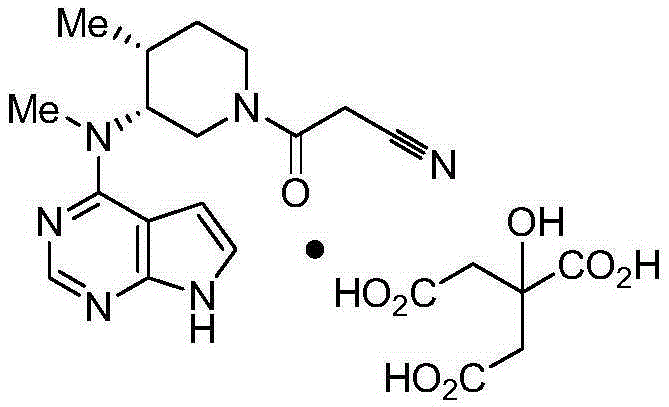
Determination method for content of tofacitinib citrate and related substances of tofacitinib citrate by reversed phase high-performance liquid chromatography
A reversed-phase high-efficiency liquid phase and tofacitinib technology, which is applied in the field of medicine, can solve problems affecting baseline noise and drift, pressure instability, and easy generation of bubbles, etc., to reduce the requirements of instruments, stabilize pressure, and facilitate experimental operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
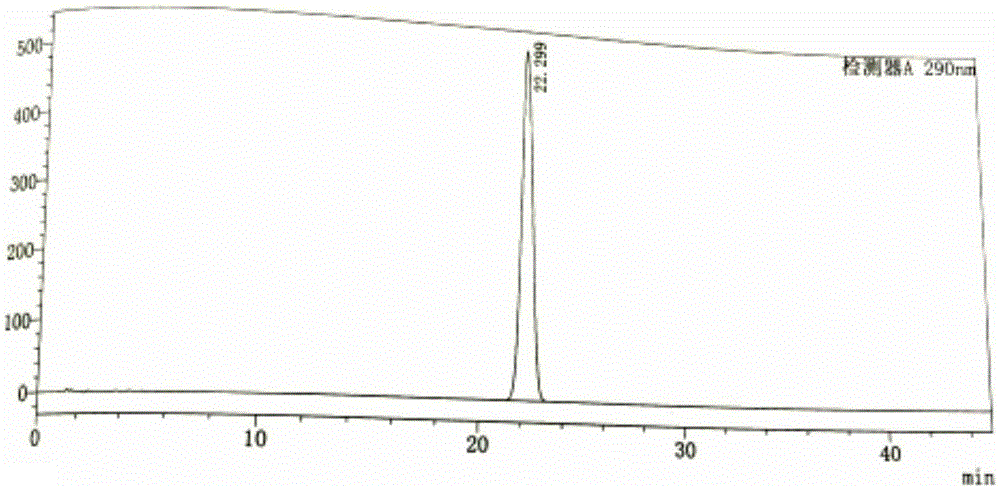
[0031] Example 1: Determination of reversed-phase high-performance liquid chromatography of tofacitinib citrate content
[0032] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use 0.02mol / L potassium dihydrogen phosphate solution (containing 0.2% triethylamine, adjust the pH value to 5.2 with phosphoric acid)-acetonitrile ( 83:17) is the mobile phase; the detection wavelength is 290nm; the column temperature is 30°C. The number of theoretical plates is not less than 3000 based on the peak of tofacitinib.
[0033] Get the tofacitinib citrate test sample, accurately weighed, dissolve and quantitatively dilute with methanol-water (90:10) and make the test sample solution containing about 1mg of tofacitinib citrate in every 1ml, Precisely measure 10 μl of the sample solution to be tested and inject it into the liquid chromatograph, and record the chromatogram; another tofacitinib citrate reference substance is taken for de...
Embodiment 2
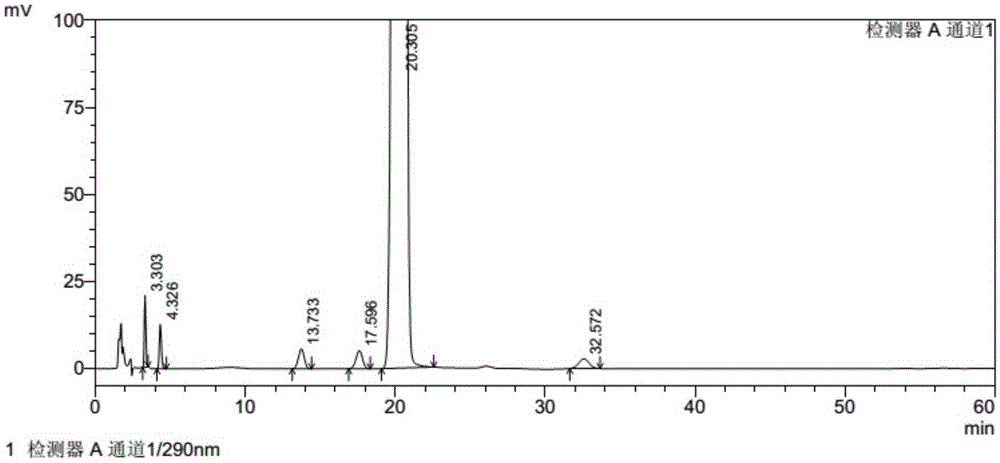
[0034] Embodiment 2: Determination of related substances of tofacitinib citrate by reversed-phase high-performance liquid chromatography:
[0035] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use 0.02mol / L potassium dihydrogen phosphate solution (containing 0.2% triethylamine, adjust the pH value to 5.2 with phosphoric acid)-acetonitrile ( 83:17) is the mobile phase; the detection wavelength is 290nm; the column temperature is 30°C. The number of theoretical plates is not less than 3000 based on the peak of tofacitinib, and the tailing factor is less than 1.5.
[0036] Get the tofacitinib citrate test sample described in Example 1, accurately weighed, dissolve and dilute with methanol-water (90:10) and make about containing tofacitinib citrate 5mg in every 1ml solution, as the test solution. Accurately measure an appropriate amount of the test solution, dilute with methanol-water (90:10) to make a solution containin...
Embodiment 3
[0047] Embodiment 3 verifies the following items of assay
[0048] a) Instrument precision test
[0049]Take about 20mg of tofacitinib citrate, accurately weigh it, put it in a 20ml volumetric flask, add methanol-water (90:10) to the near scale, ultrasonically treat it, take it out, let it cool to room temperature, add methanol-water (90:10 :10) to scale, shake up, filter, get the continued filtrate, as need testing solution. Precisely measure 10 μl of the test solution, inject it into the liquid chromatograph, record the chromatogram, repeat the injection 6 times, and calculate the RSD value of the peak area. The results are shown in Table 2 below.
[0050] Table 2 Results of precision test of content determination instrument
[0051]
[0052] Conclusion: The RSD is 0.07%, indicating that the precision of the instrument is good.
[0053] b) Recovery rate test
[0054] Take tofacitinib citrate working reference substance 15.80, 15.77, 15.85, 19.76, 19.90, 20.45, 23.96,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com