Method for detecting tofacitinib chiral intermediates and enantiomer thereof
A technology of chiral intermediates and enantiomers, which is applied in the field of analytical chemistry, can solve problems such as the lack of methods for detecting optical isomers and the inability to effectively separate compounds, and achieve stable and reliable results, high sensitivity and resolution , good repeatability and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
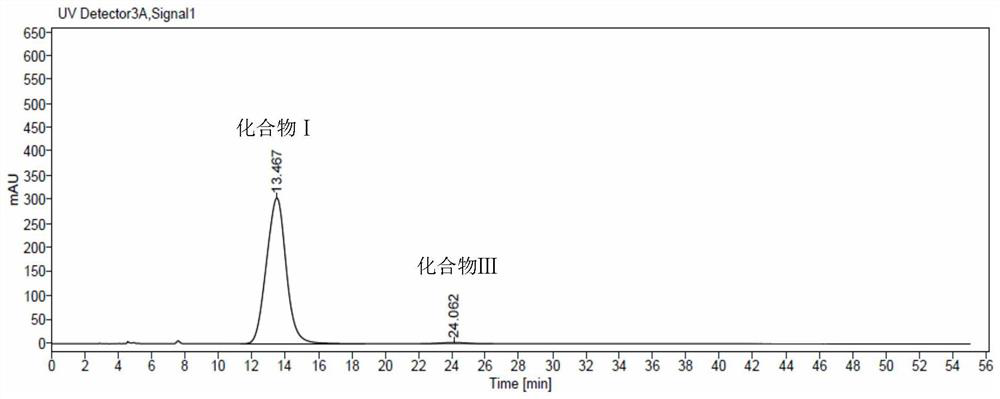
Embodiment 1
[0041] 1 Chromatographic conditions:
[0042] Chromatographic column: CHIRALPAK AD-H (250mm×4.6mm, 5μm)
[0043] Detection wavelength: 277nm
[0044] Column temperature: 30°C
[0045] Flow rate: 1ml / min
[0046] Injection volume: 20μl
[0047] Diluent: mobile phase
[0048] Mobile phase: n-hexane-isopropanol-methanol-diethylamine-trifluoroacetic acid (70:20:10:0.1:0.1)
[0049] Chromatogram acquisition time: isocratic elution at least 40min
[0050] 2 Methods and results
[0051] 2.1 Solution preparation
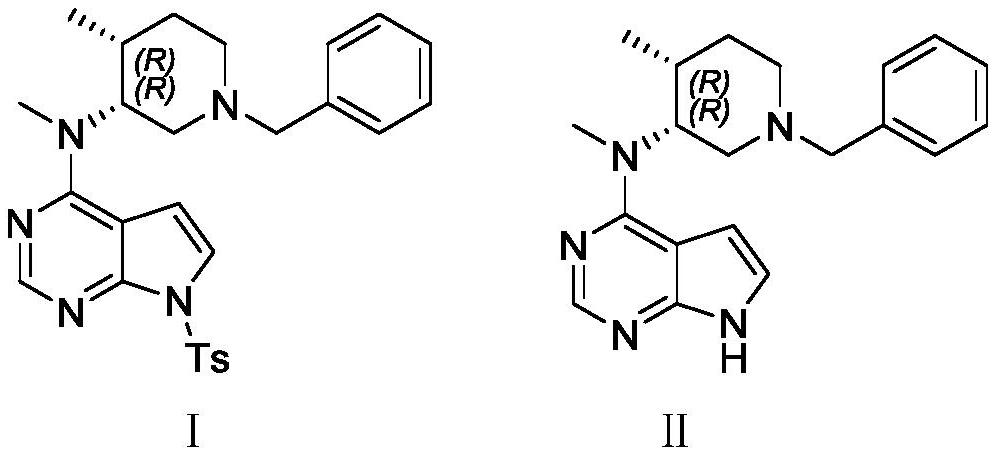
[0052] Preparation of the test solution: take an appropriate amount of tofacitinib citrate chiral intermediate Ⅰ sample, dilute with a diluent to make 1ml of a solution containing about 0.5 mg, as the test solution.
[0053] Control solution preparation: Accurately take 1ml of the test solution and place it in a 100ml measuring bottle, add diluent to dilute to the mark, shake well, and obtain the control solution.
[0054] 2.2 System suitability
[0055] Take tofacit...
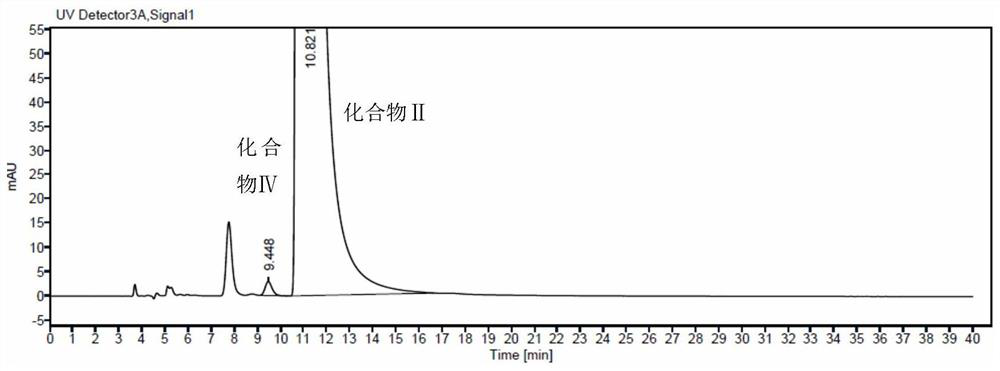
Embodiment 2
[0065] 1 Chromatographic conditions:
[0066] Chromatographic column: Kromasil 5-Amycat (250mm×4.6mm, 5μm)
[0067] Detection wavelength: 287nm
[0068] Column temperature: 30°C
[0069] Flow rate: 0.8ml / min
[0070] Injection volume: 20μl
[0071] Diluent: mobile phase
[0072] Mobile phase: n-hexane-isopropanol-methanol-diethylamine-trifluoroacetic acid (70:20:10:0.1:0.1)
[0073] Chromatogram acquisition time: isocratic elution at least 40min
[0074] 2 Methods and results
[0075] 2.1 Solution preparation
[0076] Preparation of the test solution: an appropriate amount of tofacitinib citrate chiral intermediate II sample was diluted with a diluent to make a solution containing about 0.5 mg in 1 ml as the test solution.
[0077] Control solution preparation: Accurately take 1ml of the test solution and place it in a 100ml measuring bottle, add diluent to dilute to the mark, shake well, and obtain the control solution.
[0078] 2.2 System suitability
[0079] Tofac...
Embodiment 3
[0089] 1 chromatographic column: Kromasil 5-Amycat (250mm * 4.6mm, 5 μm) replaces CHIRALPAKAD-H (250mm * 4.6mm, 5 μm) in embodiment one, all the other are the same as embodiment one,
[0090] 2 conclusions:
[0091] Under the chromatographic conditions, the chiral intermediate I of tofacitinib citrate and its enantiomers can be completely separated. Research standard technical requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com