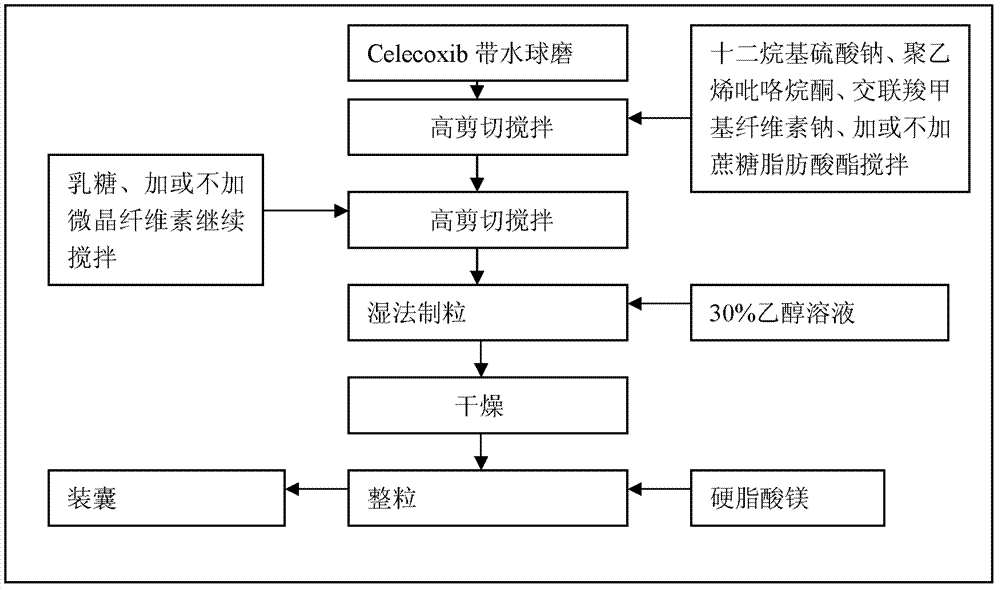
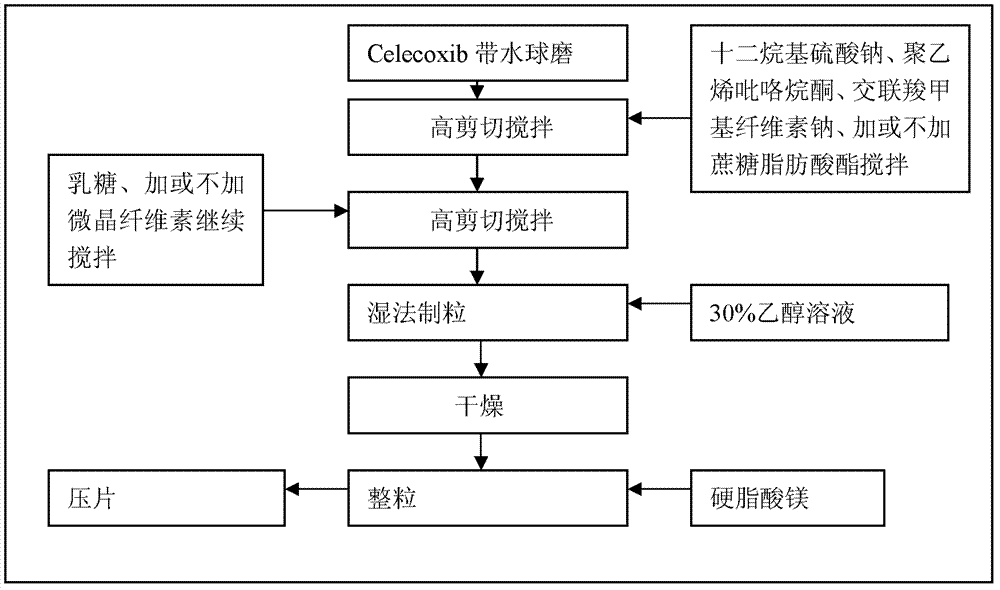
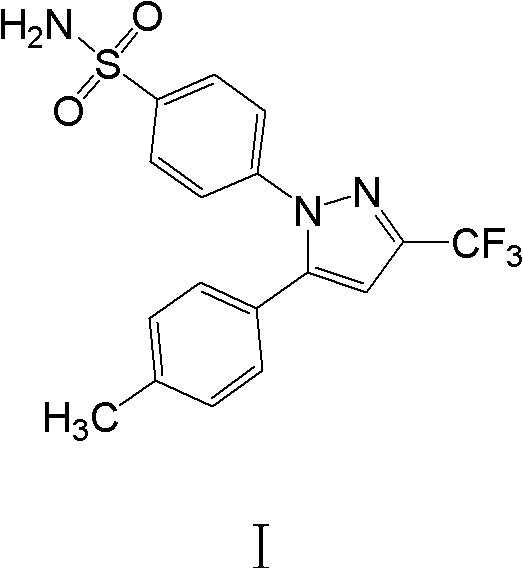
Celecoxib composition, and preparation method and application thereof
A technology of celecoxib and composition, which is applied in the field of orally releasable pharmaceutical composition and its preparation, can solve problems such as increase in tablet hardness, reduce renal side effects, reduce gastrointestinal toxicity, and reduce induction The effect of asthma attack
Inactive Publication Date: 2013-03-06
TIANJIN INSTITUTE OF PHARMA RESEARCH
View PDF4 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0013] (5), the hardness of tablet increases;
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0070] Capsules are prepared with the following composition:
[0071]
Embodiment 2
[0073] Capsules are prepared with the following composition:
[0074]
Embodiment 3
[0076] Capsules are prepared with the following composition:
[0077]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Login to View More
Abstract
The invention provides a celecoxib composition which contains one or a plurality of dose units releasable through oral administration. Each dose unit contains 50 to 500 mg of a mixture of a celecoxib D97 particle and one or a plurality of medicinal excipients. The composition can be used for treating or preventing diseases caused by COX-2.
Description
technical field [0001] The invention belongs to the technical field of medicine, and in particular relates to an orally releasable pharmaceutical composition containing an active component Celecoxib (Celebrex, trade name) and a preparation method and application thereof. Background technique [0002] In 1994, G.D Searle Company of the United States applied for a patent in China, the publication number is CN1141630, and the compound 4-[5-(4-methylphenyl-3-(trifluoromethyl-1H-pyrazole-1) was reported in the patent -yl] benzenesulfonamide (celecoxib), and claimed a class of 1,5-diarylpyrazoles and salts thereof and the method for preparing said compound. Celecoxib has the following structure: [0003] [0004] The patent reports that the compound can be used to treat inflammation and diseases related to inflammation. The formulations are in oral dosage forms such as tablets and capsules. [0005] 1998 Simon et al. in Arthritis and Rheumatology, Vol. 41, No. 9, September 19...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/635A61P19/04A61P29/00A61P19/02A61P9/00A61P9/10
Inventor 梅林雨罗振福张晓东郑志超高晶
Owner TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com