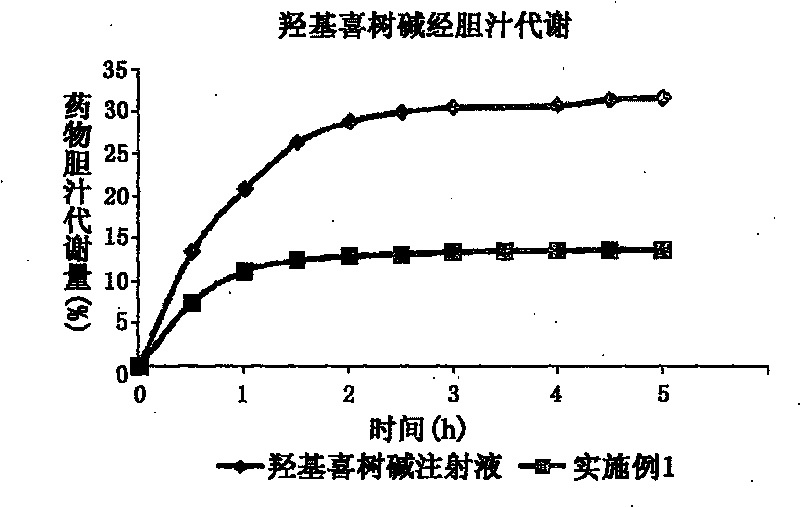
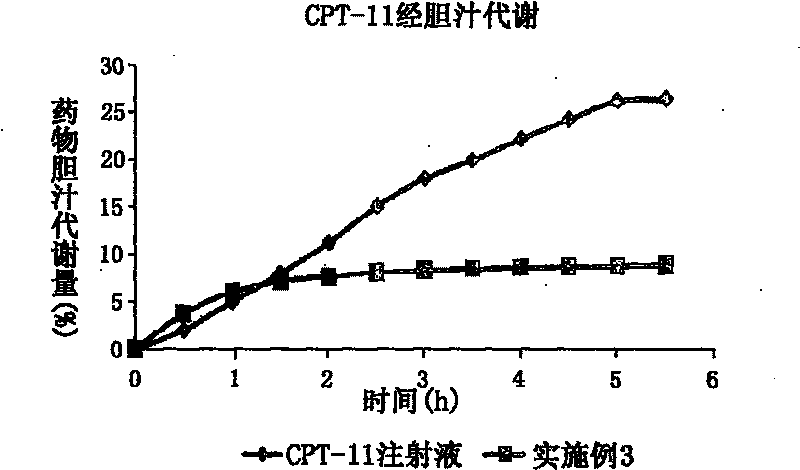
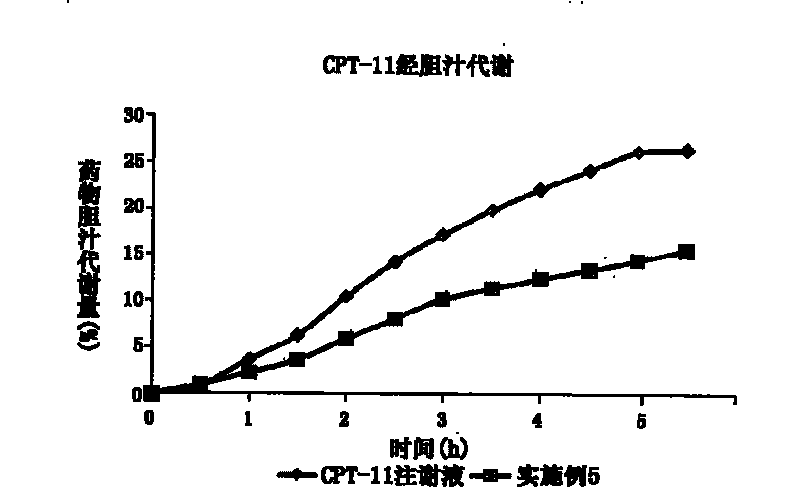
Novel type nanometer particle preparation capable of reducing gastroenteritic toxicity of camptothecin medicines
A nanoparticle and camptothecin technology, applied in the field of medicine, can solve problems such as difficult to obtain effects, achieve the effect of reducing excretion and increasing anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
[0074] Preparation method: Take the prescription amount of hydroxycamptothecin, glyceryl monostearate, medium chain triglyceride (MCT), soybean lecithin, olive ene, add appropriate amount of ethanol to dissolve, spin evaporate to dry organic solvent, vacuum dry, 80 ℃ heating and dissolving as an oil phase. Sodium oleate was dissolved in water for injection and heated to 80°C as the water phase. Under stirring at 80°C, drop the water phase into the oil phase while hot, and pass through N 2 Colostrum was prepared in a high-shear homogenizer under the condition of 2 Under the condition of high pressure homogeneous treatment, the hydroxycamptothecin nanoparticles are obtained after cooling, and then freeze-dried to obtain the hydroxycamptothecin nanoparticles freeze-dried preparation.
[0075] Average particle size (Particle size) = 112.4 ± 31.1nm; polydispersity coefficient (PI) = 0.114
Embodiment 2
[0077]
[0078]
[0079] Preparation method: Weigh hydroxycamptothecin, limonene, soybean lecithin and stearic acid according to the prescription amount, heat to (80±5)°C under nitrogen, then add glycerol and Poloxamer- 188 aqueous solution to make coarse milk; under the condition of (80±5)°C and nitrogen flow, the milk was homogenized for 5 times under the pressure of 4114 MPa on the high-pressure homogenizer, and after filling with nitrogen, it was cooled rapidly to form hydroxycamptothecin nanoparticles, and separated After packaging, freeze-drying, the hydroxycamptothecin nano-particle freeze-drying preparation is obtained.
[0080] Particle size=201.0±15.9nm; PI=0.102
Embodiment 3
[0082]
[0083] Preparation method: Weigh the prescription amount of CPT-11, glyceryl monostearate, zedoary oil, Tween80, and egg yolk lecithin, add appropriate amount of ethanol to dissolve, spin evaporate to dryness to remove organic solvent, dry in vacuum, heat and dissolve at 80°C as the oil phase. Sodium oleate was dissolved in water for injection and heated to 80°C as the water phase. Add the water phase to the oil phase under stirring at 80°C, and pass N 2 Colostrum was prepared in a high-shear homogenizer under the condition of 2 Under the condition of high-pressure homogeneous treatment, after cooling, irinotecan nanoparticles were prepared, subpackaged, freeze-dried, and stored at 4°C to obtain irinotecan nanoparticle freeze-dried preparations.
[0084] Particle size=123.6±21.0nm; PI=0.124
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com