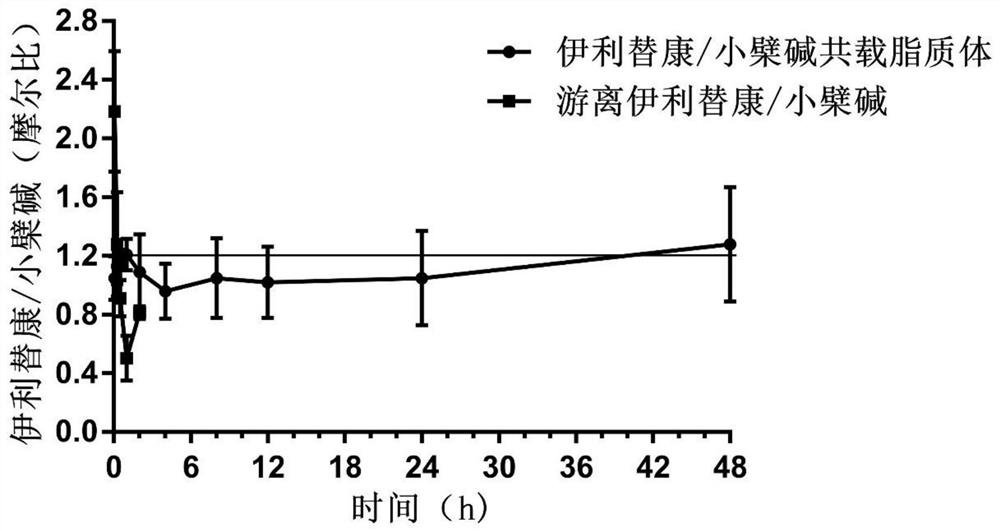
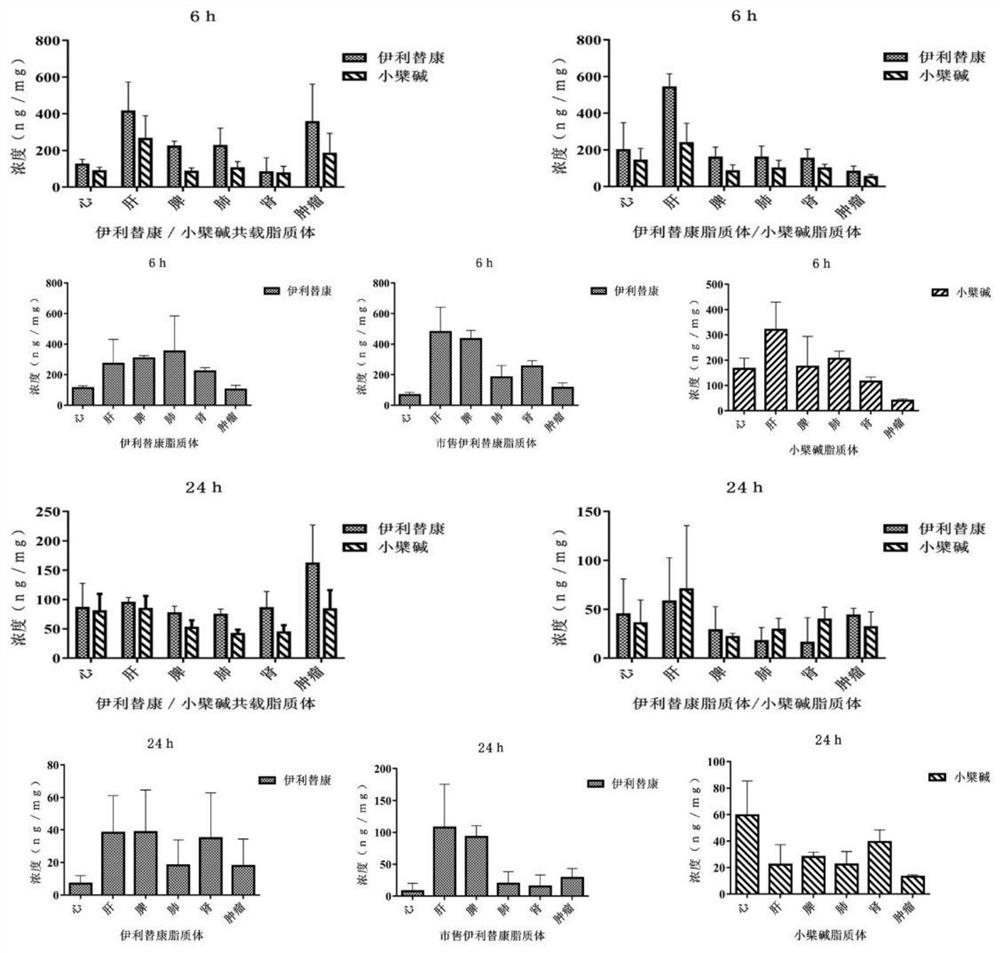
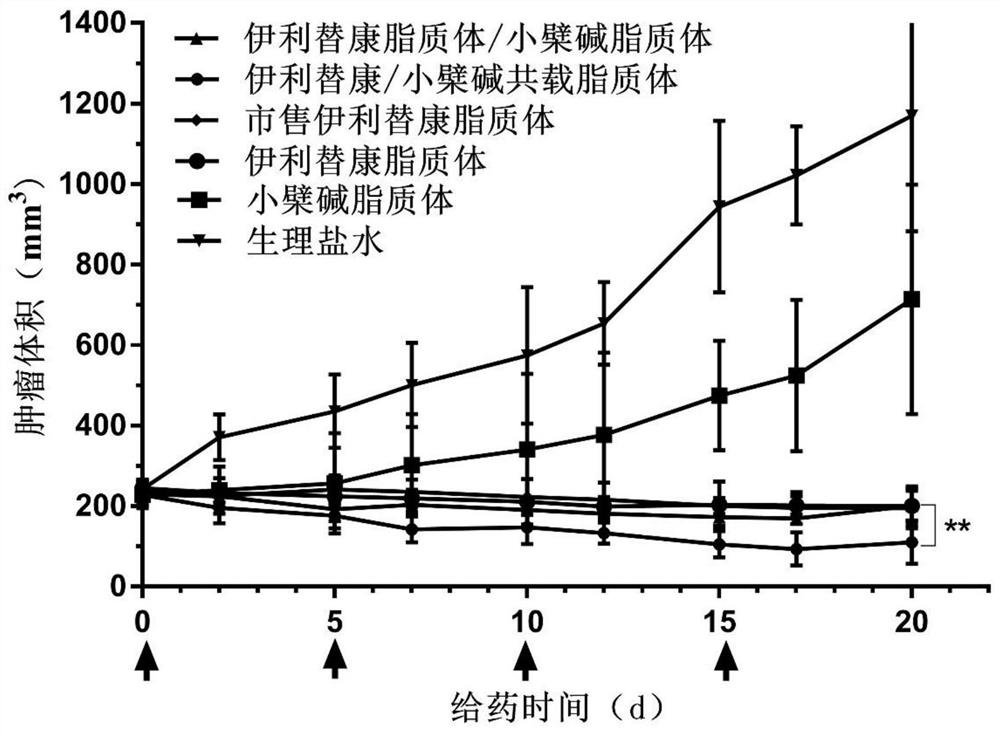
Pharmaceutical composition, pharmaceutical preparation, application and preparation method thereof for treating cancer
A technology of pharmaceutical preparations and traditional Chinese medicines, applied in the field of medicine, can solve the problems of not achieving the best therapeutic effect, rapid death of animals, vasodilation, etc., and achieve the effects of reducing gastrointestinal toxicity, uniform particle size and high encapsulation rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Weigh HSPC (150 mg), cholesterol (60 mg), DSPE-MPEG2000 (1.2 mg), dissolve them in 15 mL of chloroform, evaporate under reduced pressure at 39° C. to form a film, and place under vacuum for 12 hours to remove residual solvent. At 65°C, 10 mL of 600 mM sucrose octasulfate triethylamine salt solution was added for hydration for 30 min to form multilamellar vesicle liposomes (MLV). The MLVs were then gradually extruded through polycarbonate membranes of 0.4 μm, 0.2 μm and 0.1 μm to obtain unilamellar blank liposomes. Pass the extruded liposome sample through a Sepharose CL-4B gel column pre-equilibrated with the outer aqueous phase (20mM HEPES+150mMNaCl) to obtain a blank liposome suspension; mix 10.6 mg of irinotecan with blank The liposomes were incubated at 60 °C for 60 min, and then 5.3 mg of berberine was added. After incubation for 30 min, the drug loading was terminated in an ice-water bath. The co-loaded liposomes of irinotecan and berberine were obtain...
Embodiment 2
[0045] Example 2: This example is the same as Example 1, the difference is: 30mM sucrose+20mM histidine was used to replace 20mM HEPES+150mMNaCl, the obtained liposome irinotecan encapsulation rate was 90%, and the Alkali is 43%.
Embodiment 3
[0046] Example 3: This example is the same as Example 1, except that 20 mM HEPES+150 mM NaCl was replaced with a pH 7.0 phosphate buffer, the obtained liposome irinotecan had an encapsulation efficiency of 85%, and the Alkali is 76.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com