Statin-related drug composition as well as capsule preparation and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of narrow therapeutic index of colchicine, adverse reactions, enhanced colchicine muscle toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention also provides a kind of preparation method of capsule preparation, comprises the following steps:
[0035] A) In terms of mass fraction, mix 80-90% polyethylene glycol 1000 vitamin E succinate and 5-19% auxiliary materials to obtain mixed auxiliary materials,
[0036] The excipients include macrogol glyceride laurate, macrogol 200, macrogol 300, macrogol 400, macrogol 600, ethanol, propylene glycol and caprylic capric macrogol glyceride one or more of them;
[0037] B) by mass fraction, mixing 0.1-10% of statins and 0.01-5% of colchicine with the mixed auxiliary materials obtained in step A) to obtain the content;
[0038] C) filling the content obtained in the step B) into a capsule to obtain a capsule preparation.
[0039] In terms of mass fraction, the present invention mixes 80-90% polyethylene glycol 1000 vitamin E succinate and 5-19% auxiliary materials to obtain mixed auxiliary materials, and the present invention preferably mixes the poly...
Embodiment 1
[0049] Embodiment 1 Pharmacokinetic experiment 1
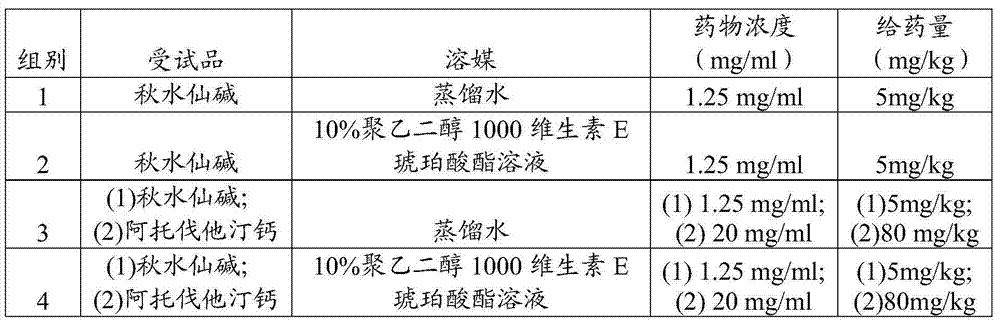
[0050] The pharmacokinetic experiment on female rats was carried out according to the formula in Table 1, and Table 1 shows the ratio of raw materials and dosage of Example 1 of the present invention. The experimental animals were female rats (180g-200g), 6 in each group, 0.3ml blood samples were collected respectively at 15min, 30min, 45min, 1h, 1.5h, 3h, 4h, 6h and 24h time points after administration of the test product solution ( Anticoagulant), the colchicine concentration in plasma was measured by HPLC, and the pharmacokinetic parameters were calculated. The experimental results are shown in Table 2. Table 2 is the pharmacokinetic experimental results of Example 1 of the present invention. The data recorded in Table 2 are the mean values ± standard deviations of the experimental data of each group.
[0051] Table 1 Raw material proportioning and dosage of the embodiment of the present invention 1
[0052]
[0053]...
Embodiment 2
[0057] Embodiment 2 pharmacokinetic experiment 2
[0058] The experimental animals were 350g~400g male rats, 5 in each group, all underwent bile duct intubation and carotid artery intubation under anesthesia (maintaining the anesthesia state during the experiment), using 4 groups of drugs as shown in Table 3, respectively. Tail vein injection. The specific administration method is: first inject polyethylene glycol 1000 vitamin E succinate (using normal saline containing 50% concentration of propylene glycol as vehicle), and then give colchicine (using normal saline containing 50% concentration of propylene glycol) after 30 minutes. as the solvent). After colchicine was administered, bile and blood were collected every 10 minutes (12 times in total), the colchicine concentration in bile and plasma was measured, and the pharmacokinetic parameters were calculated. The results are shown in Table 4, which is the pharmacokinetic parameters of colchicine in rat bile and plasma in E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com