Docetaxel and vitamin effervescent preparation and preparation method thereof
A technology for docetaxel and effervescent preparations, applied in the field of docetaxel and vitamin effervescent preparations and their preparation, can solve the problems affecting development and clinical application, poor quality stability of injections, poor water solubility of docetaxel, etc. , to achieve great development prospects, low cost, and the effect of reducing gastrointestinal toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A kind of preparation method of quick-release calcium and vitamin effervescent preparation, the preparation method of docetaxel and vitamin effervescent preparation, comprises the following steps:
[0024] (1) Docetaxel and vitamin D were weighed at a weight ratio of 1:1 to 80.
[0025] (2) Dissolving docetaxel and vitamin D in an organic solvent and heating to reflux for 0.5 to 10 hours;
[0026] (3) Concentrate the above solution under reduced pressure at -0.01~0.1Mpa vacuum pressure and temperature below 30~80°C to 5~60% of the original volume, and continue to dry at a temperature of 30~70°C to completely remove the solvent to obtain Dorsey Taxel and vitamin dispersions;
[0027] (4) Take the above dispersion, add excipients and disintegrants, and prepare docetaxel and vitamin effervescent preparations.
[0028] The acid source of the effervescent acid-base pair is any one or more of citric acid, tartaric acid, fumaric acid, citric acid monohydrate, oxalic acid, an...
Embodiment 1
[0044] The screening of embodiment 1 effervescent preparation acid source;
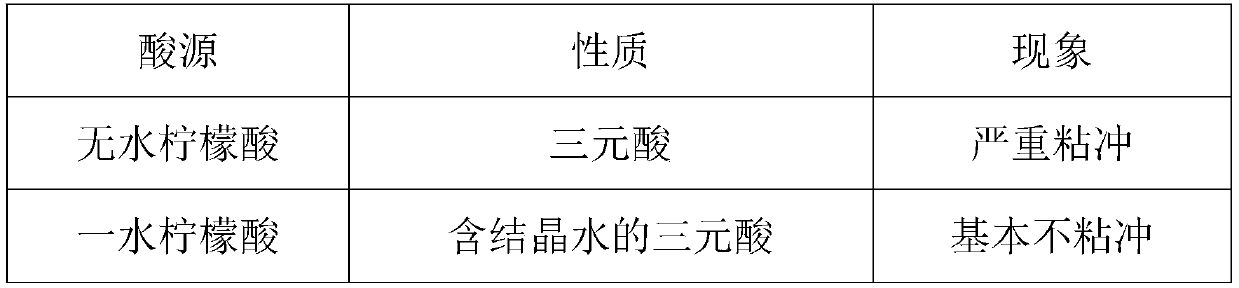
[0045] Three kinds of acid sources were selected, and through the investigation of their properties and phenomena, the results showed that citric acid monohydrate was a hydrate of crystal water, and the phenomenon of moisture absorption was improved, and the phenomenon of sticking and flushing was reduced, and citric acid monohydrate was the best. acid source.
[0046] Table 1 is the acid source screening results table.
[0047]
[0048]
Embodiment 2
[0049] The investigation of embodiment 2 effervescent preparation acid-base weight ratio;
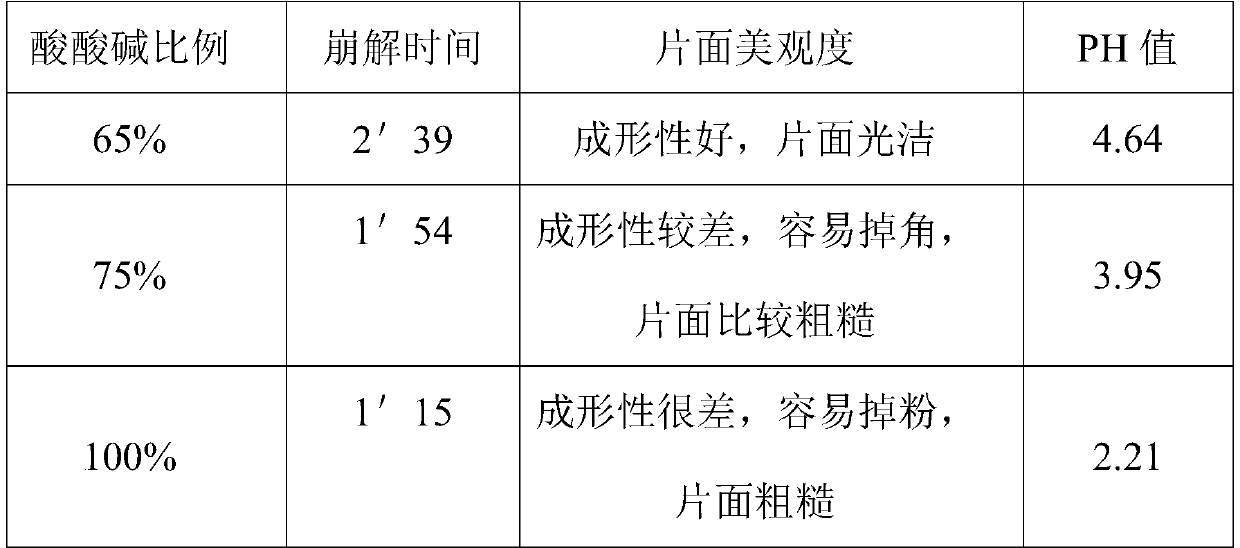
[0050] The total amount of acid and alkali was investigated and selected through the disintegration time after tablet compression, the aesthetics of the surface and the pH value. The results showed that when the total acid and alkali content was 65%, the disintegration time was short, the pH value was moderate, and the surface was smooth and beautiful.
[0051] Table 2 is a table of the investigation results of the proportion of the total amount of acid and alkali.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com