Celecoxib-containing capsule
A technology for celecoxib and capsules, applied in the field of capsules containing celecoxib, which can solve the problems of high energy consumption, poor capsule quality uniformity and stability, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
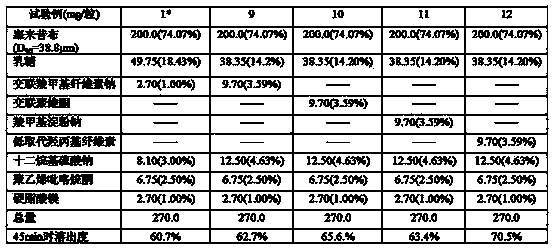
Embodiment 1
[0124] Prescription Prescription amount % by weight
[0125] Celecoxib (D 90 =25.0μm) 200.0mg 70.18%
[0126] Sorbitol 63.00mg 22.10%
[0127] Low-substituted hydroxypropyl cellulose 12.00mg 4.21%
[0128] Sodium Lauryl Sulfate 8.00mg 2.81%
[0129] Hypromellose 1.20mg 0.42%
[0130] Magnesium stearate 0.80mg 0.28%
[0131] Total 285.0mg
Embodiment 2
[0133] Prescription Prescription amount % by weight
[0134] Celecoxib (D 90=27.7μm) 100.0mg 40.00%
[0135] Mannitol 125.0mg 50.00%
[0136] Low-substituted hydroxypropyl cellulose 13.50mg 5.40%
[0137] Sodium Lauryl Sulfate 9.40mg 3.76%
[0138] Highly substituted hydroxypropyl cellulose 1.30mg 0.52%
[0139] Magnesium Stearate 0.80mg 0.32%
[0140] Total 333.0mg
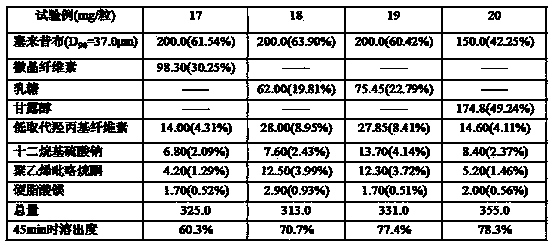
Embodiment 3
[0142] Prescription Prescription amount % by weight
[0143] Celecoxib (D 90 =50.0μm) 200.0mg 56.50%
[0144] Mannitol 44.00mg 12.43%
[0145] Lactose 44.00mg 12.43%
[0146] Low-substituted hydroxypropyl cellulose 18.50mg 5.25%
[0147] Sodium Lauryl Sulfate 35.40mg 10.00%
[0148] Polyvinylpyrrolidone 10.30mg 2.91%
[0149] Magnesium Stearate 1.80mg 0.51%
[0150] Total 354.0mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com