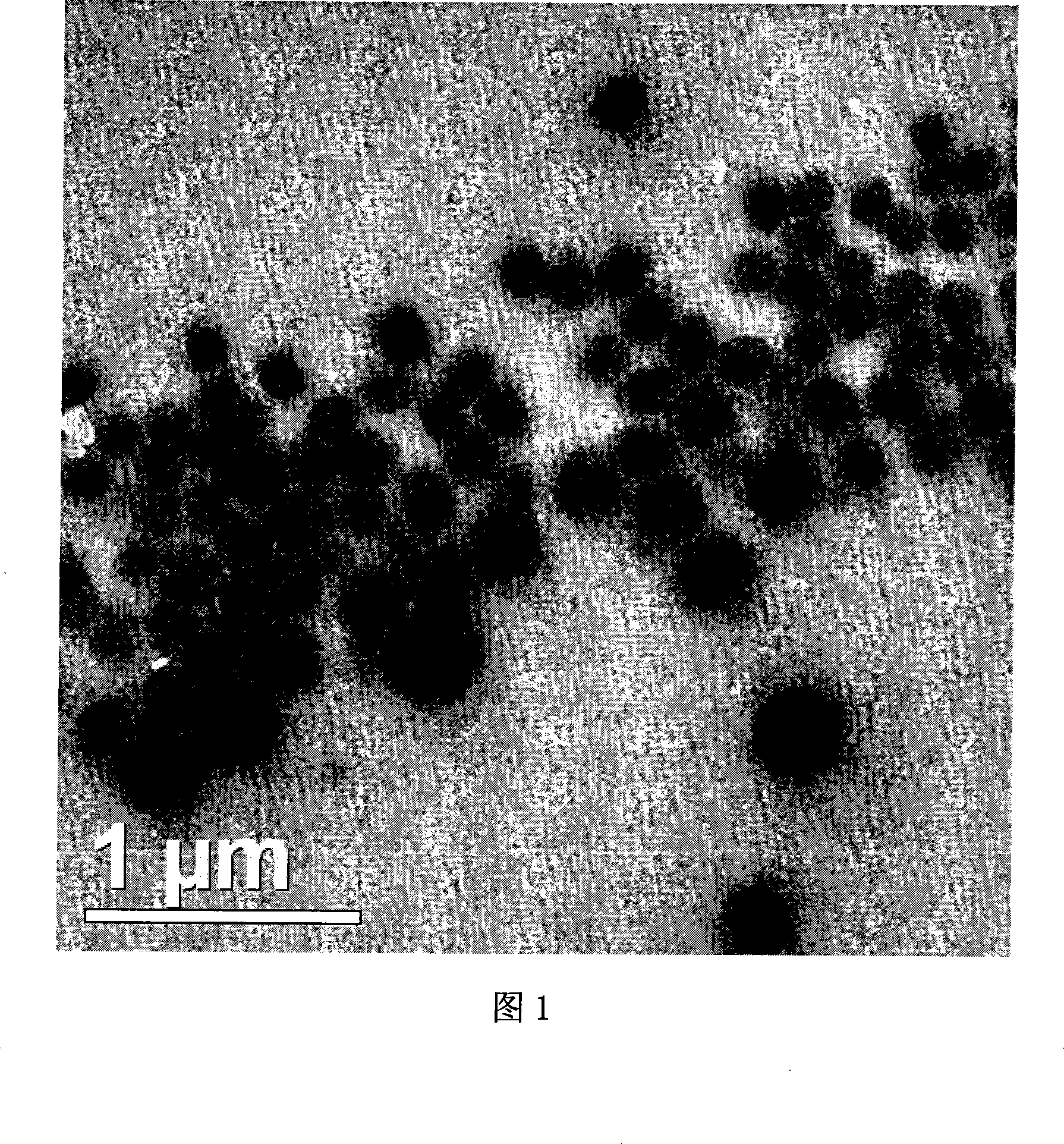
Itraconazole nanometer suspensions lyophilized compound and preparing , using method thereof
An itraconazole nanometer and nanosuspension technology, applied in the field of medicine, can solve the difficulties in the development and clinical use of itraconazole injection preparations, the poor drug-carrying performance and stability of emulsions and liposomes, and the The problems of development cost and uncertainty of curative effect, etc., achieve the effect of good reorganization and dispersion stability, broad industrialization prospects, and good storage stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation process of dichloromethane as solvent
[0029] Prepare human serum albumin (HSA) that meets the injection quality requirements into a 3% (W / V) sterile aqueous solution with water for injection, adjust the pH to 5.6 with citric acid, citric acid and some basic groups in albumin Salt formation, stabilization of albumin, stirring and addition of 1.3% dichloromethane to prepare its saturated aqueous solution. 200ml of the albumin solution sterilized in advance was mixed with 4.0ml sterile dichloromethane drug solution under vigorous stirring, and 0.6g (10% of the albumin weight) powdered sterile itraconazole (effectiveness) was dissolved in advance. valence > 99%), using a high-shear mixer to stir the mixture to prepare colostrum, add 6.0 ml of sterile ethanol dropwise, and stir evenly at high speed. Mixture colostrum is controlled preparation temperature in high-pressure homogenizer (suitably sterilized) at 50 ℃, circulates 1 time through 50bar pressure, c...
Embodiment 2
[0032] Preparation process with chloroform as solvent
[0033] Human serum albumin (HSA) meeting the injection quality requirements was prepared into 1.5% (W / V) sterile aqueous solution with water for injection, and the pH was adjusted to 7.0. 200ml of the albumin solution was mixed with 6.0ml of sterile chloroform drug solution under vigorous stirring, and 0.3g (10% of albumin weight) of powdered sterile itraconazole was dissolved in advance. Stir the mixture with a high-shear mixer to prepare colostrum, add 2.0 ml of sterile ethanol, and stir evenly. The colostrum mixture is controlled in a high-pressure homogenizer (suitably sterilized) at a temperature of 50° C., circulated twice at 200 bar, three times at 500 bar, and once at 900 bar until a nanoemulsion is obtained. Introduce it into the round bottom flask of the rotary thin film evaporator under sterile conditions, maintain the temperature of the water bath at 40 ° C, start the vacuum pump, and vacuum rotary evaporatio...
Embodiment 3
[0035] The preparation process of increasing the amount of chloroform
[0036] Human serum albumin (HSA) meeting injection quality requirements was prepared into 3% (W / V) sterile aqueous solution with water for injection, the pH was adjusted to 6.0 with acetic acid and sodium hydroxide, and the aqueous solution was saturated with 1% chloroform. 200ml of the albumin solution is mixed with 8.0ml of sterile chloroform drug solution under vigorous stirring, and the chloroform solution is pre-dissolved with 0.6g (10% of the albumin weight) powdered sterile itraconazole (potency>99 %), using a high-shear mixer to stir the mixture to prepare colostrum, add 2.0ml of sterile ethanol, and stir evenly. The colostrum mixture is controlled and prepared at 50° C. in a high-pressure homogenizer (suitably sterilized), circulated twice at 200 bar and three times at 500 bar until nanoemulsion is obtained. Introduce it into the round bottom flask of the rotary thin film evaporator under sterile...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com