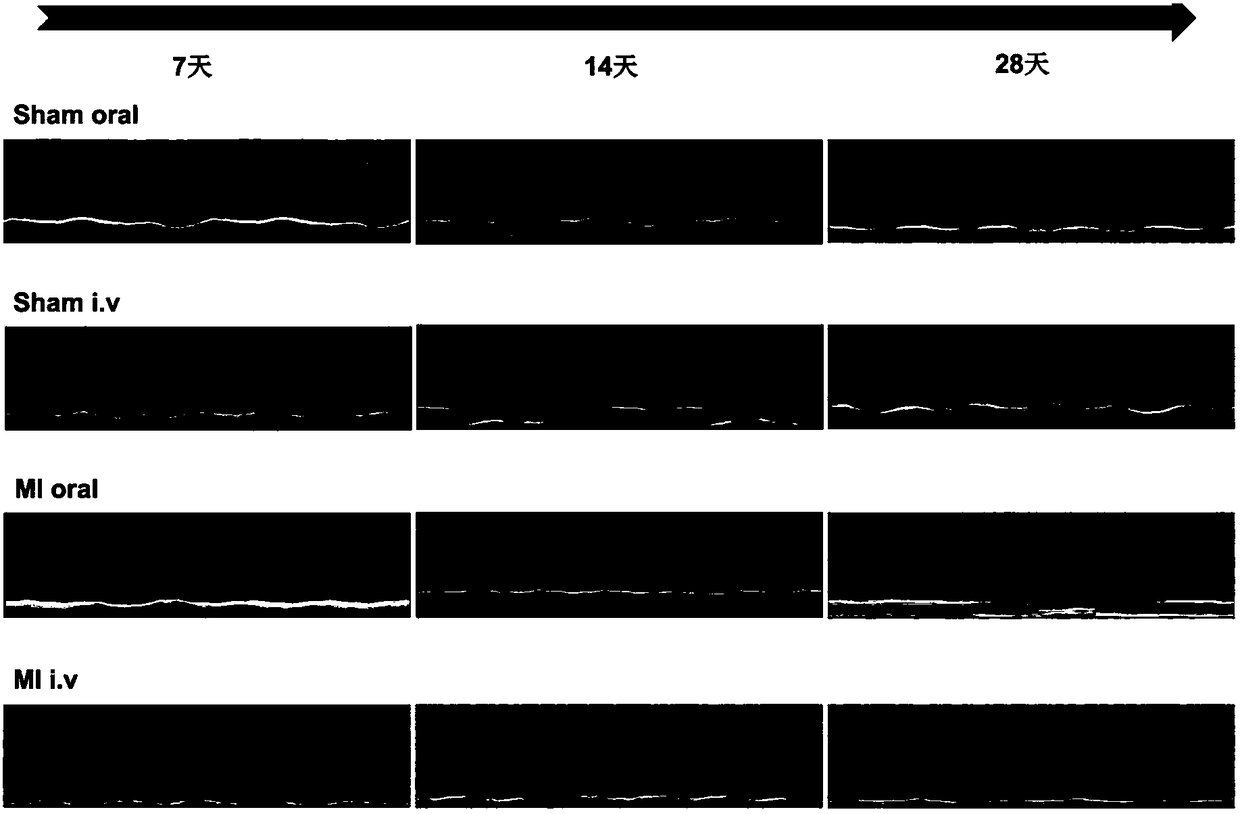
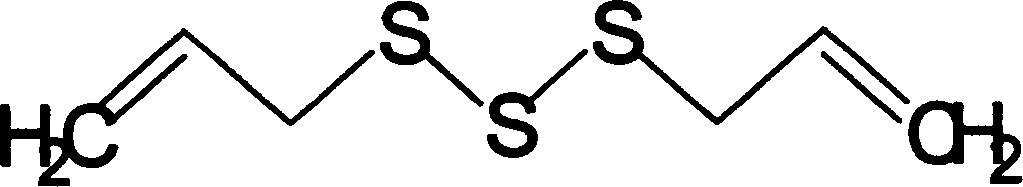
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Injection emulsion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coenzyme Q10 injection emulsion and its preparing process
InactiveCN1857239AEasy to manufactureEasy to implementOrganic active ingredientsPowder deliveryVegetable oilEmulsion
The coenzyme Q10 injection emulsion has coenzyme Q10 as the effective medicine component, and each 1000 ml emulsion contains coenzyme Q10 1-10g, vegetable oil for injection 0-200g, emulsifier 1-50g, isoosmotic regulator 5-50g, antioxidant 0.05-5g, pH regulator in the quantity of regulating pH value to 3.0-9.0, co-emulsifier 10-500g and water for injection for the rest. It provides the patient with treating medicine and essential nourishing matter. It has high physical stability and may be prepared into freeze dried preparation for further raised stability and convenient storing and transportation. The coenzyme Q10 injection emulsion has certain targeting effect, so that it has raised bioavailability and lowered toxic side effect.
Owner:YUTAI MEDICINE SCI TECH HANZHOU
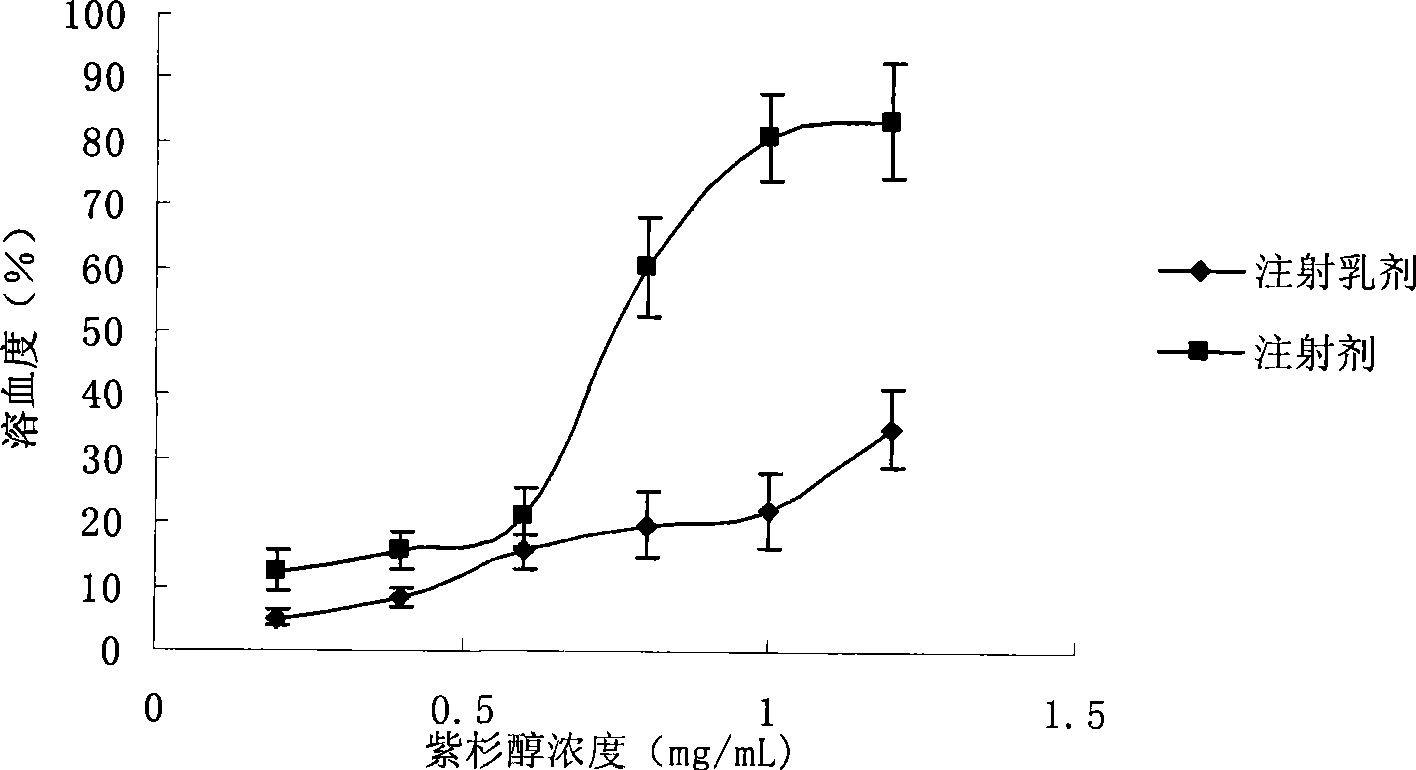
Intravenous injection emulsion of paclitaxel or polyenic taxusol
InactiveCN101433533AReduce allergic reactionsThe preparation process is simple and feasibleOrganic active ingredientsEmulsion deliveryPharmacyHaemolysis
The invention belongs to the field of traditional Chinese medicine pharmacy, in particular to a novel emulsion for intravenous injection of an anti-cancer drug taxol or polyenic taxusol, and a preparation method thereof. The emulsion consists of the taxol or the polyenic taxusol, and oil for injection, emulsion, in particular to glycol laurylhydroxystearate, and other pharmaceutically necessary auxiliary materials. Results via experiments show that the injection emulsion has excellent stability and haemolysis significantly smaller than a commercial taxol injection, and is helpful for improving administration safety and effectiveness of the taxol. The emulsion for intravenous injection can be directly used for intravenous injection, has the advantages of safe administration and small stimulation, can be easily accepted by patients, and can be used as an energy replenisher. The emulsion has the advantages of good stability, large drug loading, convenient use and simple preparation technology method, and is applicable to mass production.
Owner:FUDAN UNIV
Composite intravenous injection emulsion of glossy ganoderma spore oil and coix seed oil and method for making same
InactiveCN1990032AOvercoming the deficiency of low curative effectReduce doseDigestive systemAntiviralsSporelingInjection emulsion
Disclosed is a composite intravenous injection emulsion of glossy ganoderma spore oil and coix seed oil, which is prepared from glossy ganoderma spore oil, coix seed oil and pharmaceutically acceptable carrier suitable for preparing venous emulsions which can be solvent for injection, emulsifying agent, isotonic conditioning agent, anti-oxidant, pH regulator. The invention also discloses the process for preparing the emulsion.
Owner:TIANJIN TASLY PHARMA CO LTD
Fat emulsion of Paricalcitol, its preparation and preparation methods thereof
ActiveCN102772364ALess irritatingSafe and stable intravenous injectionPowder deliveryOrganic active ingredientsOrganic solventInjection emulsion
The invention relates to a fat emulsion of Paricalcitol. The fat emulsion comprises oil for injection, an emulsifier, a stabilizer, an isoosmotic adjusting agent, and a pH adjusting agent. Emulsion droplets have an average diameter of 50nm-1000nm. The fat emulsion can be prepared into injection emulsions, freeze-dried emulsions or capsules. Specifically, organic solvents cannot be employed as injections so as to reduce stimulation on blood vessels during injection and avoid injection package material impurity leaching caused by organic solvents, thus ensuring product quality and safety, and reducing the difficulty of product quality control.
Owner:CHONGQING HUAPONT PHARMA
Injection emulsion containing breviscapinum and preparation method thereof
InactiveCN1875981AOvercoming the disadvantages of instability and inconvenient clinical applicationImprove bioavailabilityOrganic active ingredientsMetabolism disorderEmulsionInjection emulsion
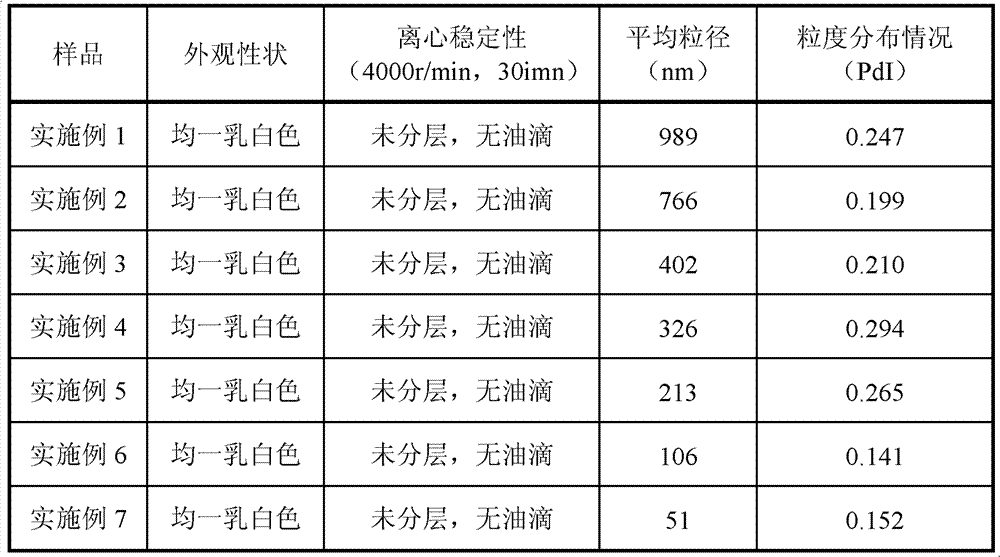
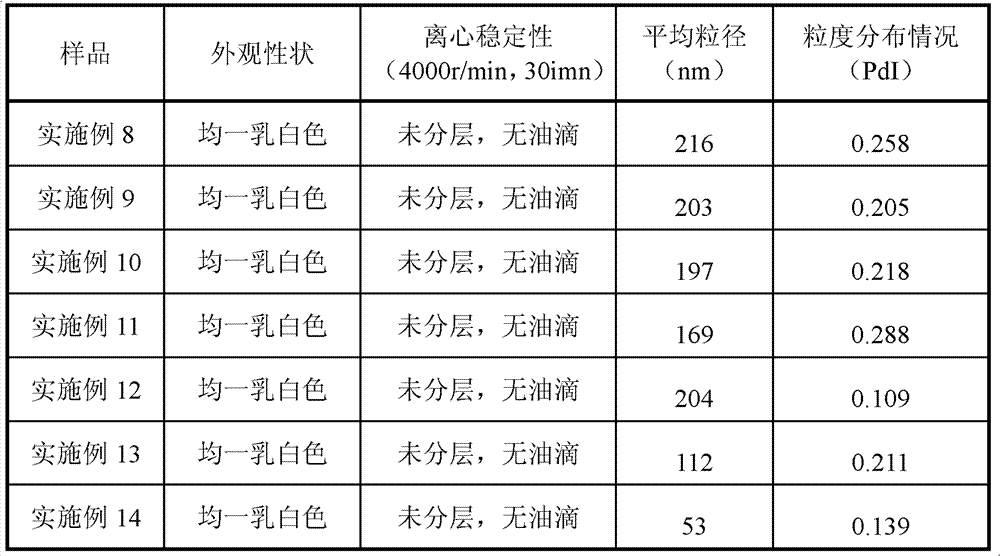
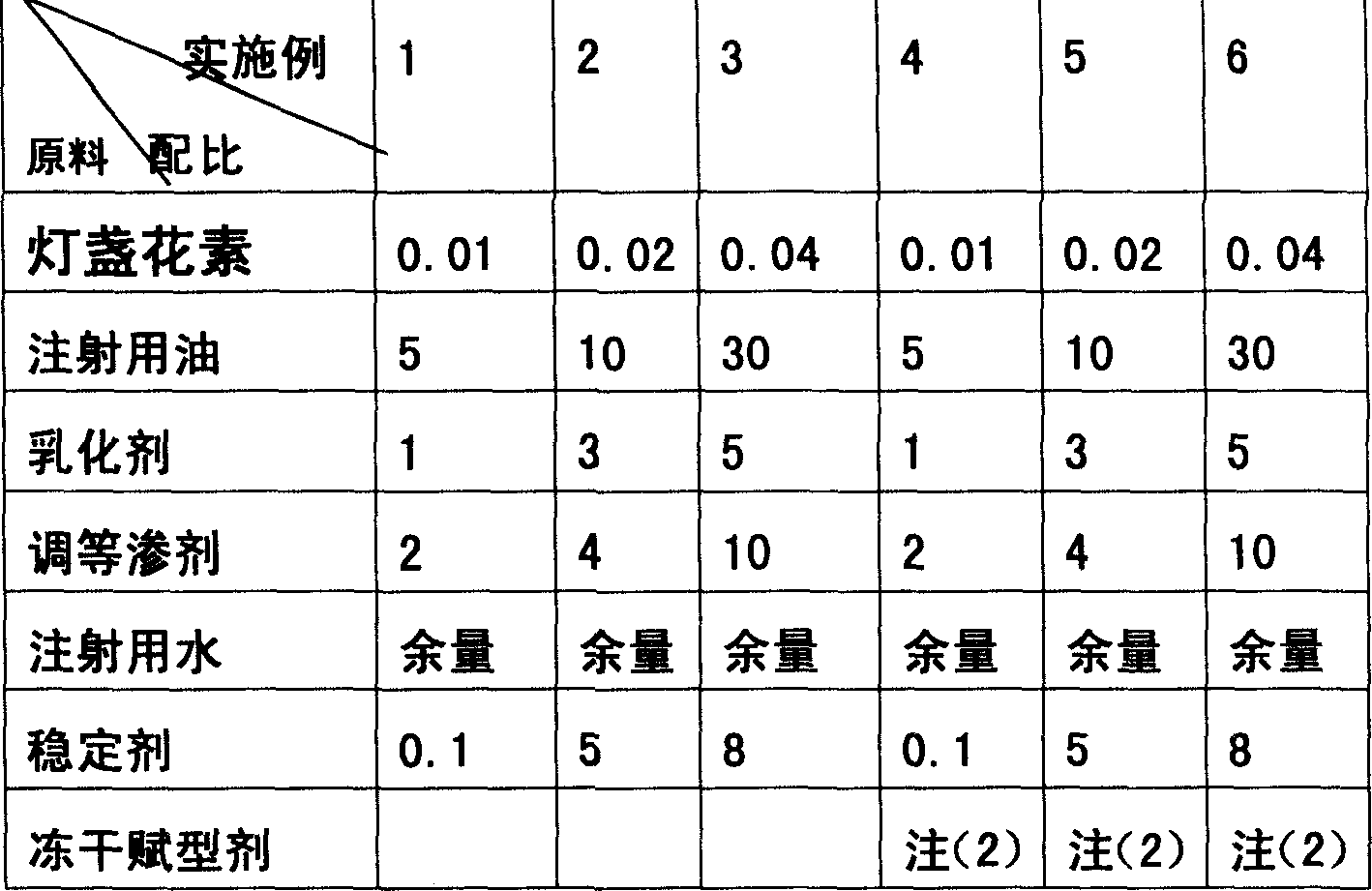
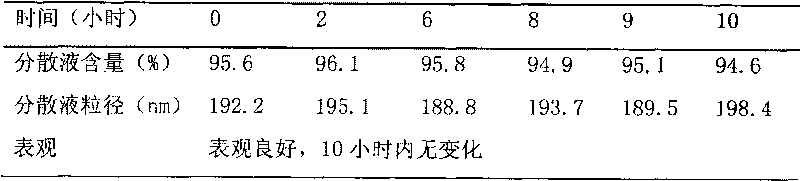
The invention relates to an emulsion injection containing Breviscapine and its preparing process, which is prepared from the following raw materials (by weight portion): Breviscapine 0.01-0.04%, oil for injection 5-30%, emulsifying agent 1-5%, isotonic agent 2-10%, stabilizer 0.1-8% and balancing water for injection. The preparation process comprises the steps of proportioning, making oily phase mixture, making initial milk, emulsifying and sterilizing.
Owner:石家庄大为生物技术有限公司
Camptothecin medicament injection solution and injection and preparation method thereof
InactiveCN101708156AGood dispersionSolve problems that are difficult to make into injectionsOrganic active ingredientsEmulsion deliverySolubilityMedication injection
The invention relates to the technical field of medicaments, in particular to a camptothecin medicament injection solution and an injection and a preparation method thereof. The invention provides a camptothecin medicament injection which is good in stability, high in curative effect and low in toxicity and consists of the camptothecin medicament injection solution and a disperse medium, wherein the camptothecin medicament injection solution consists of a medicament active component, a stabilizing agent, a pH value regulator and an injection solvent. The medicament active component of the camptothecin medicament injection solution is a camptothecin medicament mainly existing in the form of a lactonic ring, which can improve the curative activity of the medicament and reduce toxic side effects; and the disperse medium is an injection emulsion, which can improve the dispersion degree of the camptothecin medicament and solve the problem that the camptothecin medicament is difficult to be prepared into the injection due to poor water solubility. The camptothecin medicament injection solution and the injection emulsion are packed and then stored respectively, and are mixed uniformly before use for intravenous administration, so the stability of the medicaments stored for a long time can be improved. The invention provides an intravenous injection which has low toxicity and is save and convenient to store for camptothecin medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Asarone injection emulsion and its preparing method
The present invention relates to an asarone injection emulsion and its preparation method. Its composition includes active component asarone and medicinal auxiliary material which can be selected from injection vegetable oil, emulsifying agent and isoosmotic regulating agent. Said asarone injection emulsion can be used for resisting bacteria, relieving inflammation, stopping cough, expelling phlegm, relieving asthma, relieving convulsion and resisting epilepsy, etc.
Owner:曾列丹
Antibiotic oil-water double suspension type injection emulsion for livestock and preparation method thereof
ActiveCN102119923AAvoid drug resistanceLess irritatingAntibacterial agentsImmunological disordersIntramuscular injectionInjection emulsion
The invention discloses an antibiotic oil-water double suspension type injection emulsion for livestock. The effective antibiotic components of the antibiotic oil-water suspension type injection emulsion are respectively suspended in an oil phase and a water phase, the water phase is in the outer layer and the oil phase is enveloped therein. In the invention, based on the metabolic characteristics of the effective antibiotic components in a target animal body, the effective antibiotic components of the end products of the new preparation are distributed in an equivalent or nonequivalent mode in different oil and water carriers to form the double suspension type emulsion. After the double suspension type emulsion is administered by intramuscular injection, the antibiotics present a split type secondary release characteristic, thus achieving quick acting and long acting combination. The invention has the characteristics that: (1) the preparation process is relatively simple, the materials can be acquired easily, and the preparation process is particularly suitable for large-scale production; (2) medicaments with different amounts of carriers can be designed according to the pharmacokinetic characteristics of different target animals on different antibiotics, thus achieving quick acting and long acting combination and the effect on treating infection, and the medicament is particularly suitable for clinical application; and (3) the carriers of the double suspension type emulsion can carry health raw materials for animals for preparing compound health products for animals.
Owner:HUNAN AGRICULTURAL UNIV +2
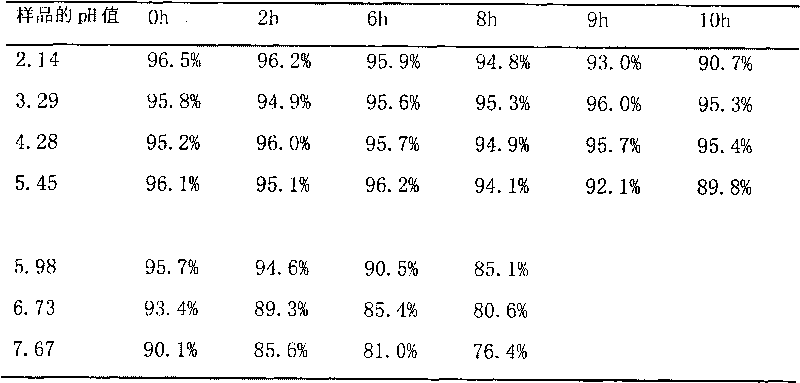
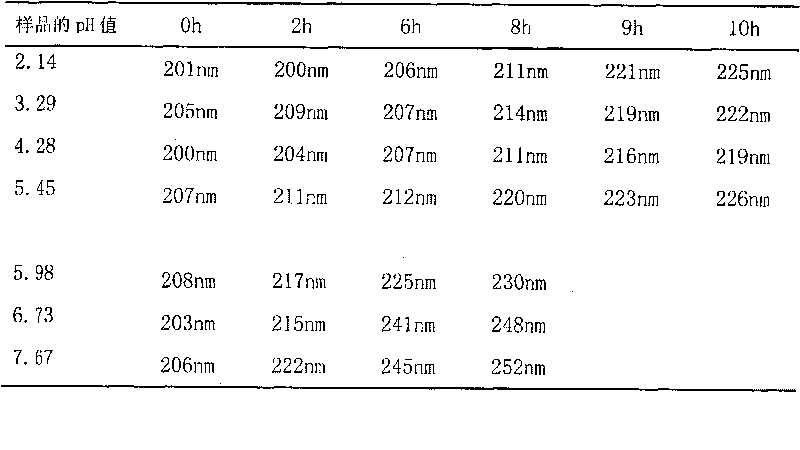
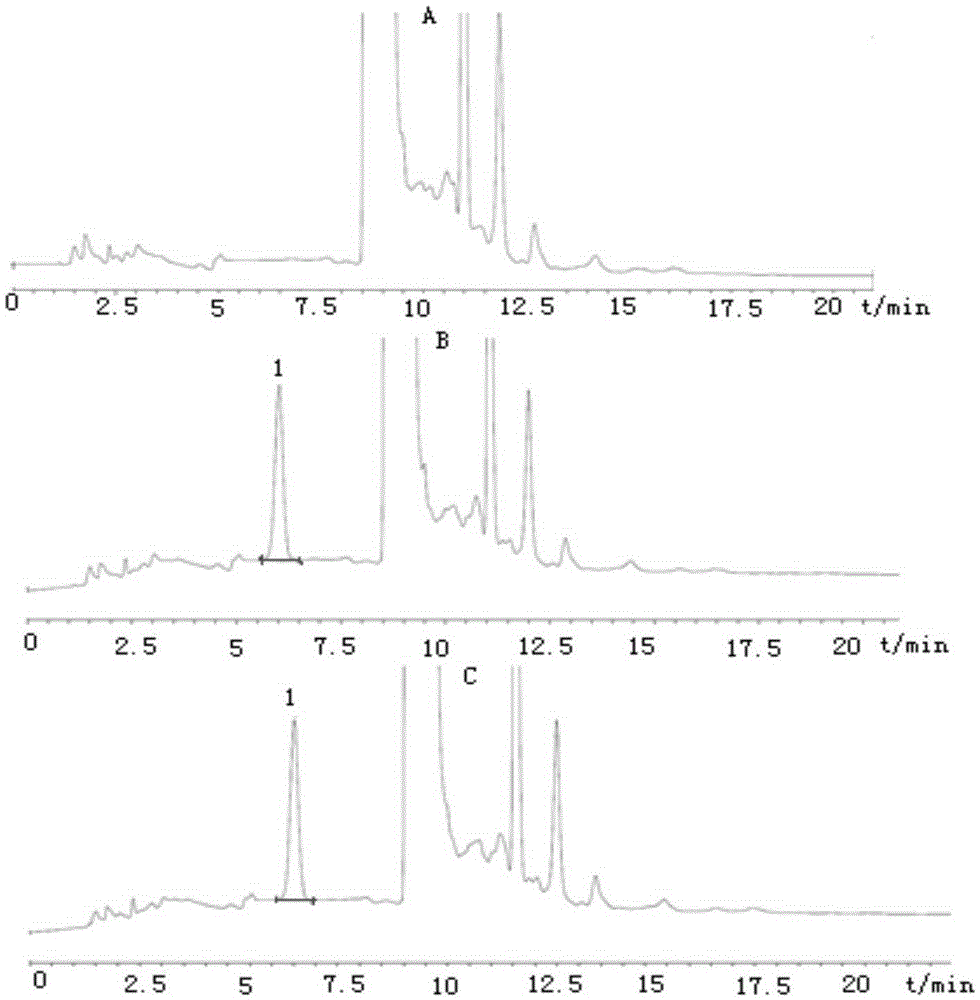
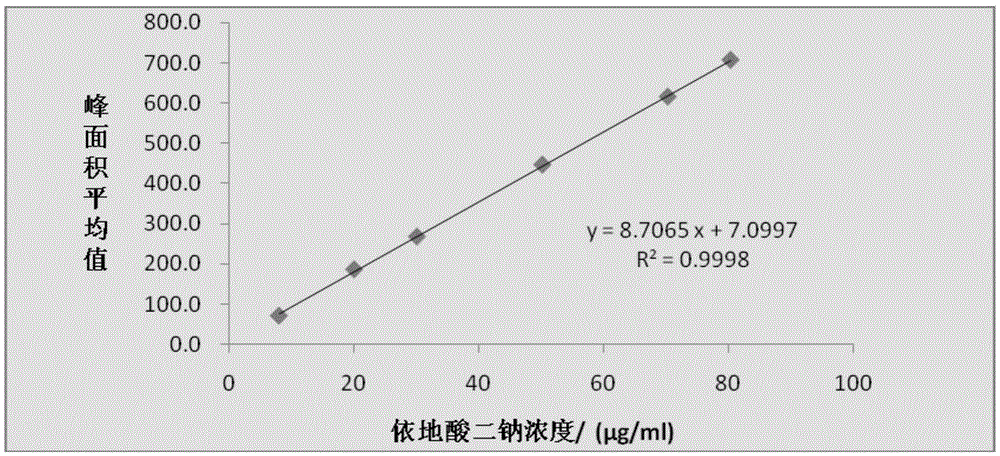
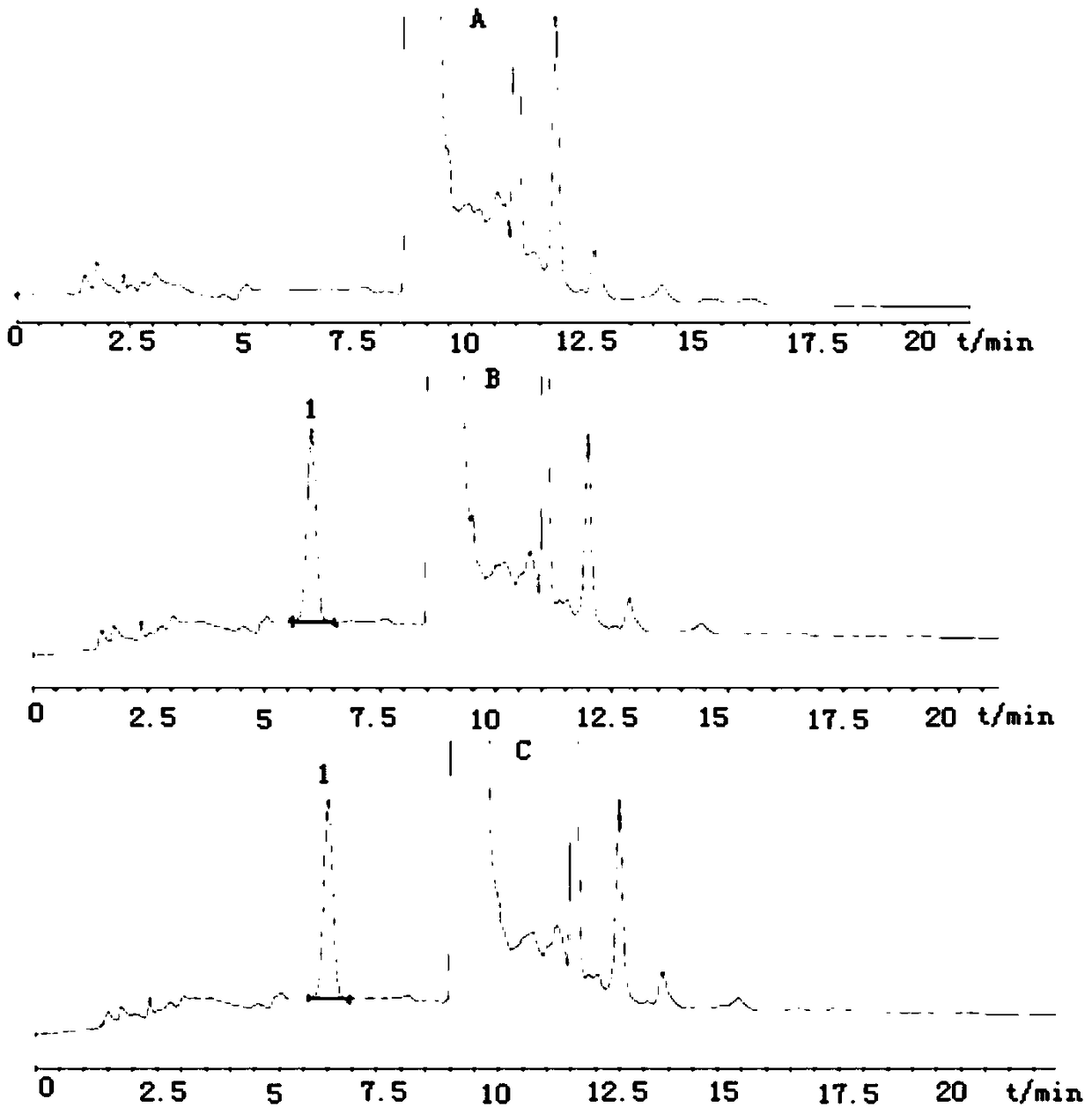
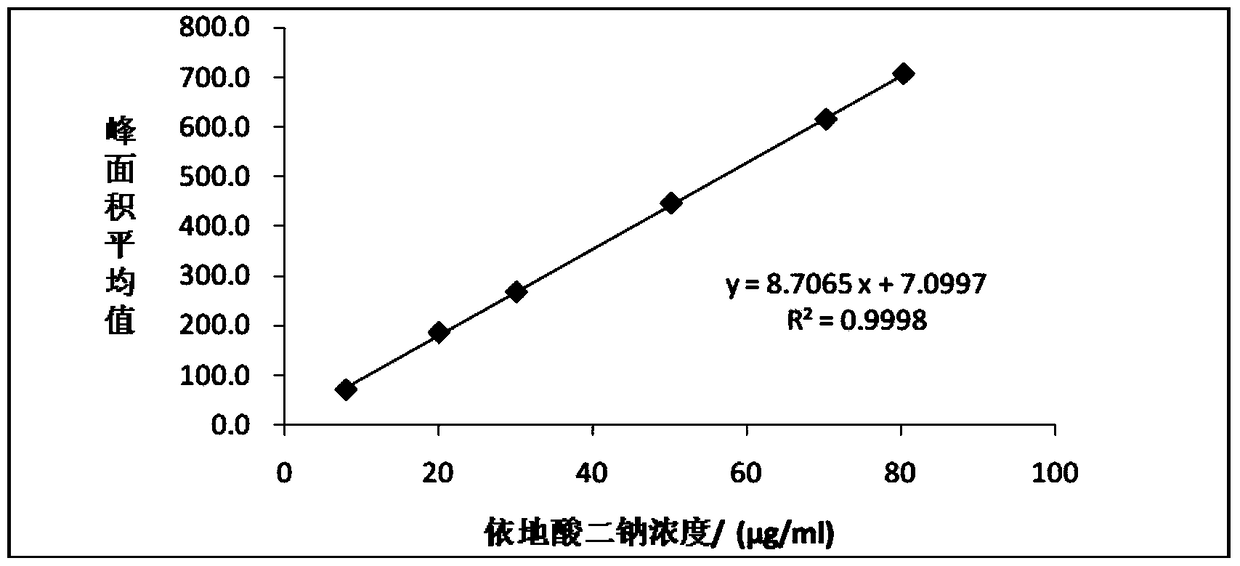
Method for detecting disodium edetate in clevidipine butyrate injection emulsion
The invention provides a preparation method of a sample used for HPLC detection of disodium edetate in a clevidipine butyrate injection emulsion. The preparation method comprises the following steps: adding an isopropanol and n-hexane mixed liquid into the clevidipine butyrate injection emulsion, shaking the mixed liquid and the emulsion, centrifuging the obtained solution, taking obtained water phase, and adding a metal ion salt solution able to complex edetic acid to form a complex in order to form a sample solution. The invention also provides a method for detecting disodium edetate in the clevidipine butyrate injection emulsion. The detection method is an HPLC method. An oil phase and a water phase in the clevidipine butyrate injection emulsion are effectively separated to make disodium edetate dissolved in the water phase be accurately detected. The detection method has the advantages of high detection sensitivity, good precision and high specificity, and is an effective method for strictly controlling the content of disodium edetate in the clevidipine butyrate injection emulsion.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
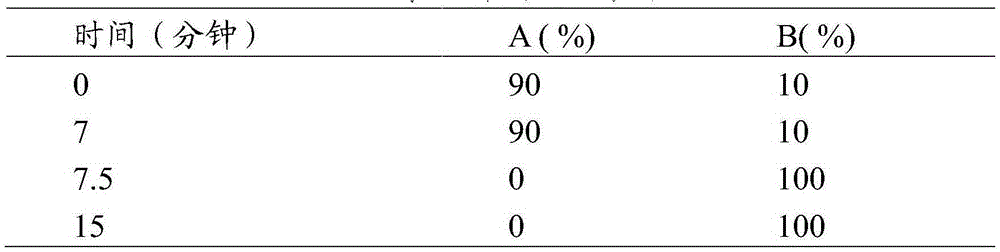
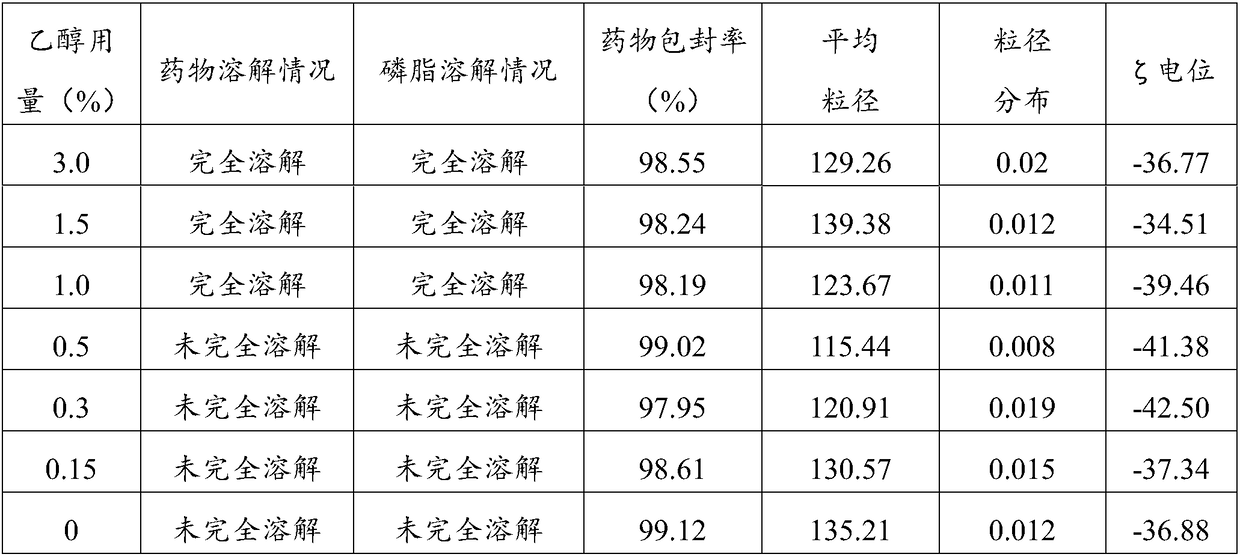
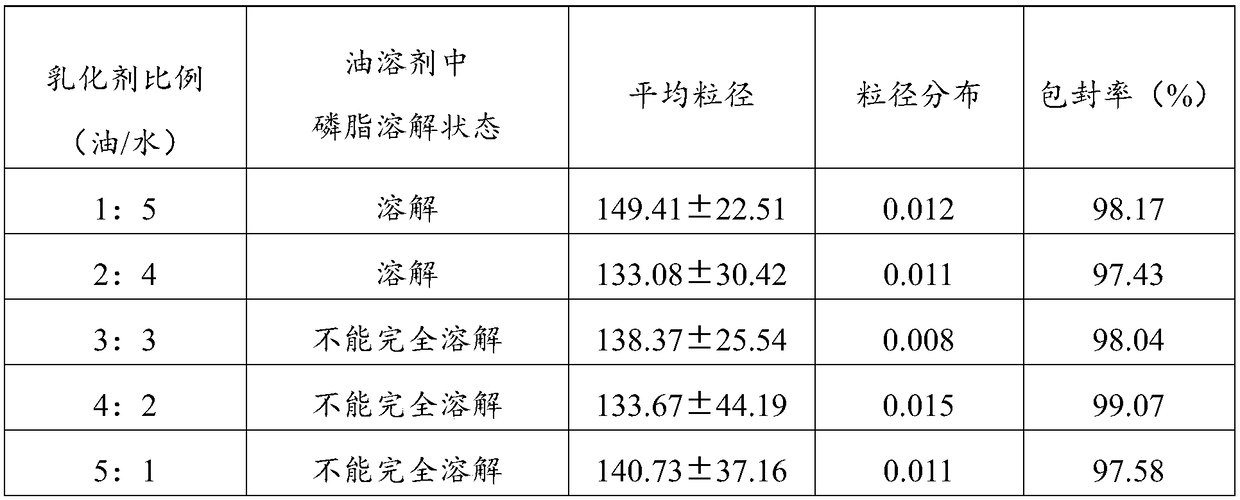
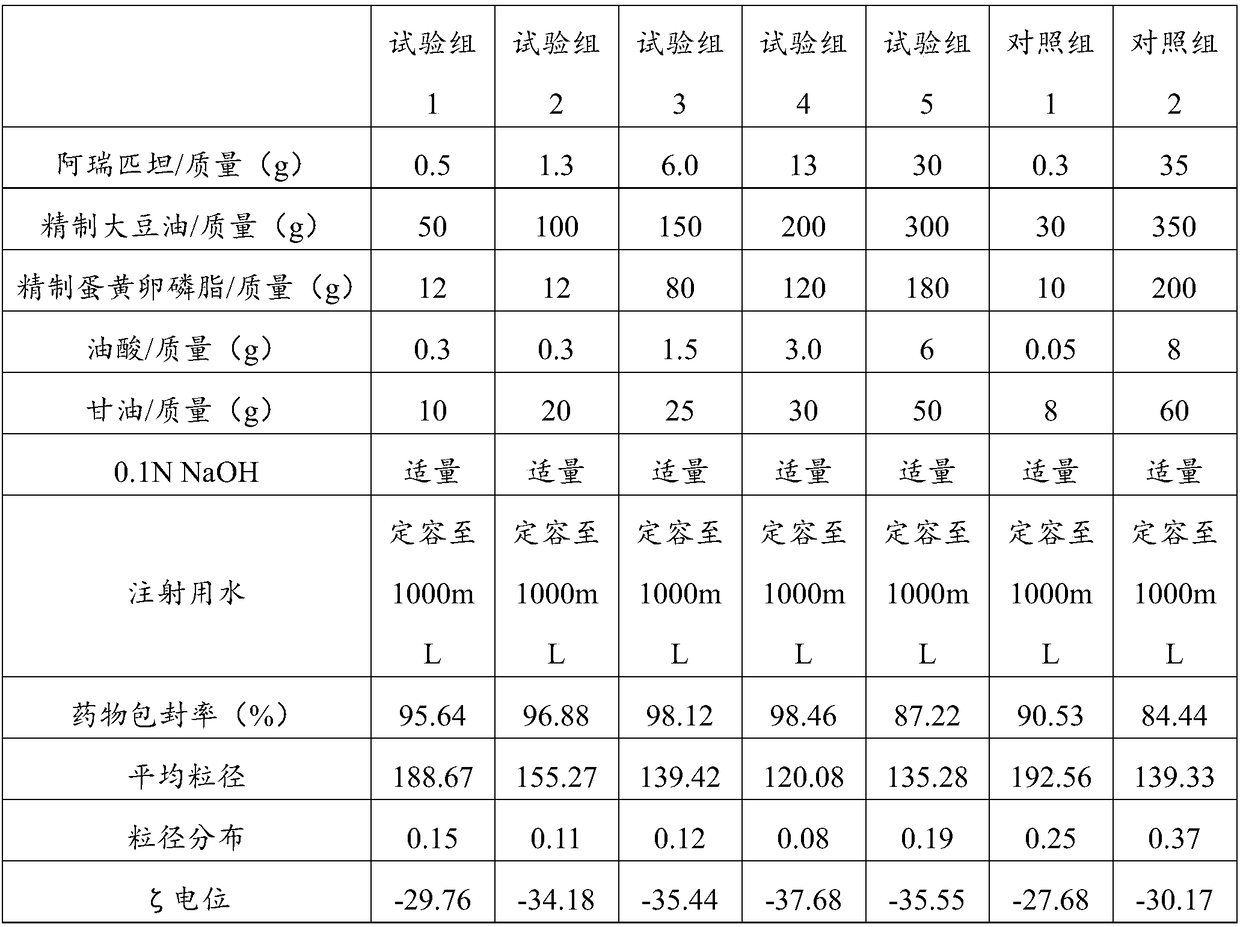
Aprepitant intravenous injection emulsion as well as preparation method and application thereof
PendingCN109364023AImprove liquidityNo hanging phenomenonOrganic active ingredientsDigestive systemHigh dosesMarketed products
The invention relates to aprepitant intravenous injection emulsion as well as a preparation method and an application thereof, and belongs to the technical field of pharmaceutics. The aprepitant intravenous injection emulsion is prepared from components in percentage by mass as follows: 0.05%-3% of aprepitant, 5%-30% of an oil phase solvent, 1.2%-18% of an emulsifier, 0.03%-0.6% of a stabilizer and 1%-5% of an isoosmotic adjusting agent, the injection emulsion further contains a pH regulating agent and the balance of water for injection, and pH value of the injection emulsion is 5.5-8.0. The aprepitant intravenous injection emulsion contains no low-carbon chain alcohol such as ethanol and the like, meanwhile, the quality requirement for a marketed product can be met by technological innovation and adjustment, and clinical use safety of the aprepitant intravenous injection emulsion is greatly improved; direct intravenous injection is utilized during clinical using, and dilution is not needed; the aprepitant intravenous injection emulsion can be used for treating acute and tardive nausea and vomiting caused by high-dose cis-platinum combined high-dose sensitizing cancer chemotherapy,and nausea and vomiting in early stage and middle stage of tumor chemotherapy are reduced.
Owner:GUANGZHOU HANFANG PHARMA
Garlicin injection emulsion and its preparing method
InactiveCN1615828AReduce contentImprove thermal stabilityAntibacterial agentsOrganic active ingredientsSolubilityWater based
The garlicin emulsion injection is a kind of water base dispersion; has dispersed phase of average size smaller than 1 micron, and with garlicin in 0.5-2.5 weight portions, emulsifier for injection 0.2-7.5 weight portions, co-emulsifier 0-3 weight portions, oil phase dispersing medium 5-20 weight portions and isosmotic agent 0.5-3 weight portions; and contains garlicin in 1-20 mg / ml. The preparation process includes mixing garlicin, emulsifier, co-emulsifier, oil phase dispersing medium and isosmotic agent and adding water for injection; ultrasonic crushing to form initial emulsion and further emulsification in high pressure emulsifying machine to form water base dispersed phase of average size smaller than 1 micron; regulating pH to 5-7, microporous film filtering, packing and sterilizing to obtain garlicin injection. The present invention solves the problem of poor water solubility and less stimulation to blood vessel and raised garlicin stability.
Owner:HUAZHONG UNIV OF SCI & TECH +1
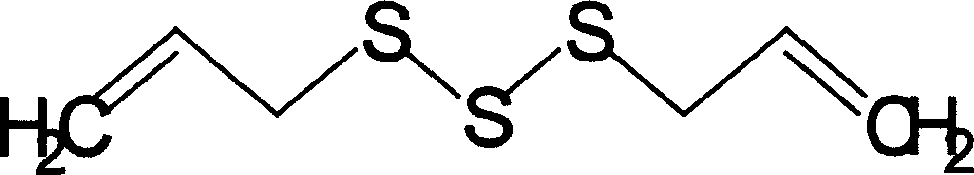
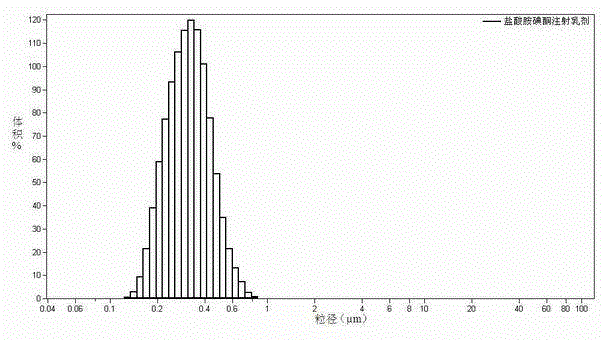
Amiodarone hydrochloride injection emulsion and preparation method thereof
ActiveCN103054799AImprove stabilitySimple manufacturing processOrganic active ingredientsEmulsion deliveryInjection emulsionCo solvent
The invention provides an amiodarone hydrochloride injection emulsion and a preparation method thereof. The amiodarone hydrochloride injection emulsion contains 0.05-0.5% of amiodarone hydrochloride, 10-30% of oil for injection, 0.5-5% of emulsifier, 2-8% of osmotic pressure modifier, and an approximate amount of pH regulator and water for injection, and also contains 0.001-0.01% of antioxidant and 0.01-0.1% of stabilizer. The preparation method comprises the following main steps of: heating an oil phase and an aqueous phase respectively; adding medicaments into the oil phase, and then adding the oil phase into the aqueous phase (or adding the aqueous phase into the oil phase); stirring to obtain initial emulsion; regulating the pH value; homogenizing the initial emulsion through a high-pressure homogenizer; and sterilizing through high temperature steam, thereby obtaining a finished product. The emulsion for injection is uniform in granularity, stable in quality and easy for industrial production, can be used for improving the in-vivo bioavailability of the medicaments, and avoiding toxic and side effects generated by a cosolvent in a general injection, so that the controllability and safety of the clinical medication are improved greatly.
Owner:BEIJING TIDE PHARMA
Tetrandrine emulsion for injection and its preparing method
InactiveCN1813736AImprove stabilityGood orientationOrganic active ingredientsAntipyreticInjection emulsionEmulsion
The present invention discloses a tetrandrine injection emulsion for effectively curing rheumatism and tumor and relieving pain. Its composition includes 15.0-200.0g of tetrandrine, 50-300g of vegetable oil for injection, 5-20g of emulsifying agent, 20-60g of isoosmotic regulating agent and injection water adding to 1000 ml. Its preparation method includes the following steps: dissolving, heating, uniformly mixing, emulsifying, cooling, filling and sterilizing.
Owner:肖春
Paeonal injection emulsion for anti-tumor and its preparing method
InactiveCN1682753AQuick effectHigh drug contentOrganic active ingredientsAntineoplastic agentsEmulsionSide effect
The present invention relates to paeonal injection emulsion for treating tumor and is preparation process. The emulsion consists of liposoluble paeonal and water soluble glossy ganoderma polysaccharide as effective components. The paeonal injection emulsion has high and fast curative effect on tumor, high medicine content, and less toxic side effect.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Nimodipine injection emulsion and its preparing method
InactiveCN1562010AImprove stabilityLow toxicityOrganic active ingredientsNervous disorderDiseaseInjection emulsion
A nimodipine injection in the form of emulsion for treating hemorrhagic cerebrovascular diseases, hypertension, senile dementia, etc is prepared from nimodipine, plant oil for injection, emulsifier and isotonic regulator through dissolving nimodipine in said plant oil and proportionally mixing others.
Owner:SICHUAN KELUN PHARMA CO LTD
Injection agent for protecting ischemic myocardium and preparation method of injection
ActiveCN108324703AIncrease local effective concentrationIncrease local drug concentrationOrganic active ingredientsSolution deliverySide effectReperfusion injury
The invention relates to an injection agent for protecting ischemic myocardium and a preparation method of the injection. Injection emulsion is prepared from the following raw materials in parts by mass: 1 to 5 parts of N-SAHA (Suberoylanilide Hydroxamic Acid), 0.2 to 12.5 parts of emulsifying agent, 2 to 100 parts of oil for injection, 0.02 to 5 parts of solubilizer, 0.03 to 0.4 part of oleic acid, 0.4 to 12.5 parts of glycerinum and the balance of water for injection. The injection emulsion can be effectively used for increasing local drug concentration of the N-SAHA in lipophilic organs such as angiocarpy and / or tissues before cardiac interventional operation, increasing the bioavailability of the N-SAHA, reducing general side effects of the N-SAHA, realizing effective protection on myocardium under an ischemic or reperfusion injury state and reducing or avoiding the occurrence of myocardial infarction; meanwhile, a new optional dosage form is provided for application of the N-SAHAin treating cancer by the injection emulsion.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Baicalin emulsion and preparation method thereof
InactiveCN103830176AImprove stabilitySolve the defect of low oral bioavailabilityAntibacterial agentsOrganic active ingredientsInjection emulsionOral emulsion
The invention provides a baicalin oral emulsion and an injection emulsion. The emulsion contains baicalin, oil, an emulsifier and water, and the injection emulsion also contains an isoosmotic adjustment agent and the like.
Owner:QINGDAO BAICAOHUI INST OF CHINESE HERBAL MEDICINE
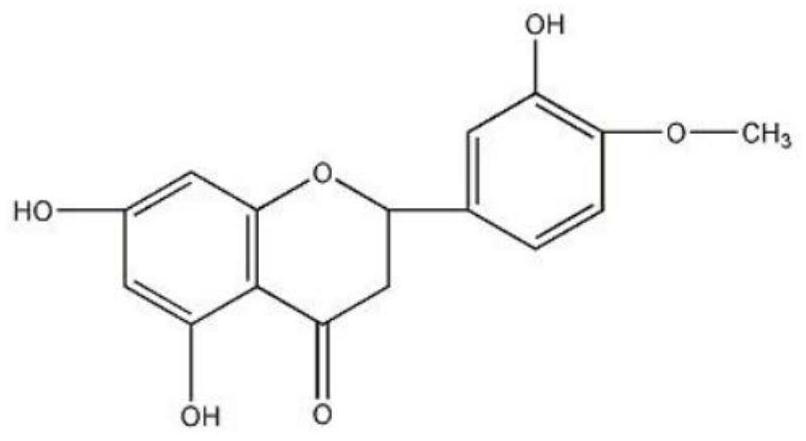
Hesperetin emulsion and preparation method thereof
ActiveCN113181114AHigh drug loadingAchieve preparationAntibacterial agentsOrganic active ingredientsInjection emulsionMedicinal chemistry
The invention relates to a hesperetin emulsion and a preparation method thereof, and belongs to the technical field of pharmaceutics. The hesperetin emulsion comprises the following components in percentage by mass: 0.3-3% of hesperetin, 10-30% of an oil phase solvent, 3.0-20% of an emulsifier, 0.03-0.6% of a stabilizer and 1-5% of an isoosmotic adjusting agent. The injection emulsion further comprises a pH regulator, a hesperetin solubilizer and the balance of water for injection, the pH value of the injection emulsion is 7.0-8.5, the hesperetin drug loading capacity is greatly improved, and the problem of rapid metabolism due to oral absorption is solved.
Owner:GUANGZHOU HANFANG PHARMA
Chinese and Western medicine compound injection emulsion used for treating bacterial disease of poultry and livestock and preparation method thereof
InactiveCN101947228AResidue reductionTo achieve the effect of sustained releaseAntibacterial agentsOrganic active ingredientsBiotechnologyWestern medicine
The invention discloses Chinese and Western medicine compound injection emulsion used for treating bacterial disease of poultry and livestock and a preparation method thereof. Each 1,000ml of injection consists of the following components: 1o to 30g of enrofloxacin, 1 to 10g of baicalin, 100 to 350ml of medical vegetable oil for injection, 5 to 20g of emulsifier, 0.1 to 1g of antioxygen, 2 to 6g of isoosmotic adjusting agent and the balance of water for injection. The preparation method comprises the following steps of: respectively weighing the vegetable oil for injection and the water for injection, sterilizing, and naturally cooling; weighing the enrofloxacin, the baicalin, the emulsifier, the isoosmotic adjusting agent and the antioxygen, dissolving in the sterilized water for injection, and heating and stirring to obtain a water phase; dissolving the emulsifier in the vegetable oil, and heating and stirring to obtain an oil phase; adding the water phase into the oil phase with stirring, emulsifying to obtain protogala, and further homogenizing; and fixing the volume to 1,000ml by using the sterilized water for injection, filling, and sterilizing to obtain the injection emulsion. The product has the advantages of sustained-release effect, high bioavailability, low administration frequency, a little medicament residue in vivo, and cost conservation.
Owner:ZHENGZHOU HOUYI PHARMA
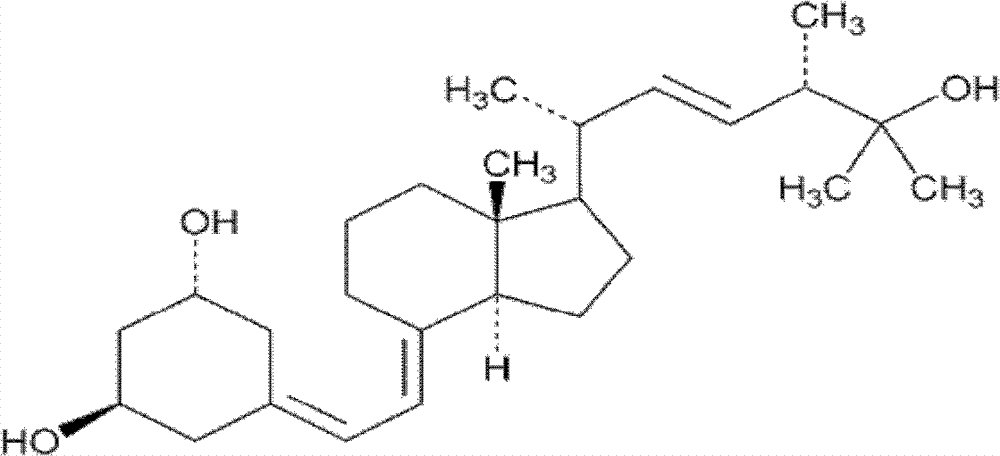
Ginkgolide emulsion for injection
InactiveCN1813825ARetain the complete structureRetain biological activityOrganic active ingredientsNervous disorderDiseaseEmulsion
The present invention discloses a bilobalide (mixture or ginkgo extract) emulsion for injection, in particular, it relates to a bilobalide (including single bilobalide A, B and C, etc.) injection emulsion for curing angiocardiopathy and cerebrovascular disease. It is made up by using bilobalide and medicinal auxiliary material according to a certain ratio through the processes of dissolving, heating, uniformly mixing, emulsifying, cooling and filling to obtain the invented product.
Owner:欧苏 +1
Fat emulsion of paricalcitol and its preparation and preparation method
ActiveCN102772364BLess irritatingReduce usageOrganic active ingredientsPowder deliveryInjection emulsionOrganic solvent
The invention relates to a fat emulsion of Paricalcitol. The fat emulsion comprises oil for injection, an emulsifier, a stabilizer, an isoosmotic adjusting agent, and a pH adjusting agent. Emulsion droplets have an average diameter of 50nm-1000nm. The fat emulsion can be prepared into injection emulsions, freeze-dried emulsions or capsules. Specifically, organic solvents cannot be employed as injections so as to reduce stimulation on blood vessels during injection and avoid injection package material impurity leaching caused by organic solvents, thus ensuring product quality and safety, and reducing the difficulty of product quality control.
Owner:CHONGQING HUAPONT PHARMA
Compound paclitaxel-oleum fructus bruceae injection emulsion and preparation method thereof
ActiveCN101361771AImprove solubilityImprove complianceOrganic active ingredientsEmulsion deliverySolubilitySide effect
The invention relates to a compound paclitaxel, namely oleum fructus bruceae emulsion for injection, and a preparation method thereof, which pertain to the field of medicine preparation, and aim at solving the technical problem of looking for a new paclitaxel emulsion for intravenous injection with large drug-loading rate, few side-effects and convenient drug administration and a preparation method thereof so as to meet the requirements of markets for the new medicine. Particularly, the new paclitaxel emulsion is prepared by the steps: after dissolving in ethanol, the paclitaxel is added with the oleum fructus bruceae to form a compound oil phase and then is prepared into the emulsion of oil-in-water type with accessories for injection, such as lecithin, water for injection and the like. In the invention, the paclitaxel is obtained by a plurality of screening experiments; the paclitaxel in the compound oil phase consisting of ethanol and the oleum fructus bruceae has very high solubility; an external water phase does not contain the paclitaxel, and therefore the emulsion of oil-in-water type prepared effectively prevents phlebitis caused by the external water phase, greatly reduces anaphylactic reaction and enhances the adaptability of patients. In addition, the preparation of the emulsion has simple prescription and does not produce sediment after being diluted; embolizing does not occur during intravenous injection, thus providing a new paclitaxel emulsion for intravenous injection in clinical treatment.
Owner:CHONGQING LUMMY PHARMA
Garlicin injection emulsion and its preparing method
InactiveCN1267090CReduce contentImprove thermal stabilityAntibacterial agentsOrganic active ingredientsWater basedSolubility
The garlicin emulsion injection is a kind of water base dispersion; has dispersed phase of average size smaller than 1 micron, and with garlicin in 0.5-2.5 weight portions, emulsifier for injection 0.2-7.5 weight portions, co-emulsifier 0-3 weight portions, oil phase dispersing medium 5-20 weight portions and isosmotic agent 0.5-3 weight portions; and contains garlicin in 1-20 mg / ml. The preparation process includes mixing garlicin, emulsifier, co-emulsifier, oil phase dispersing medium and isosmotic agent and adding water for injection; ultrasonic crushing to form initial emulsion and further emulsification in high pressure emulsifying machine to form water base dispersed phase of average size smaller than 1 micron; regulating pH to 5-7, microporous film filtering, packing and sterilizing to obtain garlicin injection. The present invention solves the problem of poor water solubility and less stimulation to blood vessel and raised garlicin stability.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Preparation process and application of tryptanthrin injection emulsion
InactiveCN102319211AReduce manufacturing costImprove preparation qualityOrganic active ingredientsAntimycoticsDiseaseLeucocythemia
The invention provides a medicament for treating leucocythemia and other tumors, colonitis and fungal infection, namely a tryptanthrin injection emulsion, and a preparation process thereof, and belongs to the fields of pharmaceutical manufacture and clinical application. The tryptanthrin injection emulsion is characterized in that: tryptanthrin has the effects of killing and inhibiting tumor cells, transplanted tumors in animals, various fungi and bacteria, an effect of inhibiting inflammation, as well as regulation and other pharmacological activities to the immunity of a body. The tryptanthrin injection emulsion prepared by the tryptanthrin for treating leucocythemia and other tumors, colonitis and fungal infection can be used for treating the leucocythemia and other tumors, colonitis, fungal infection and other diseases. The tryptanthrin injection emulsion has the advantages of stable medicament, controllable quality, high bioavailability, small administration dosage, exact curative effect, low side response, rich raw material source and low production cost.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
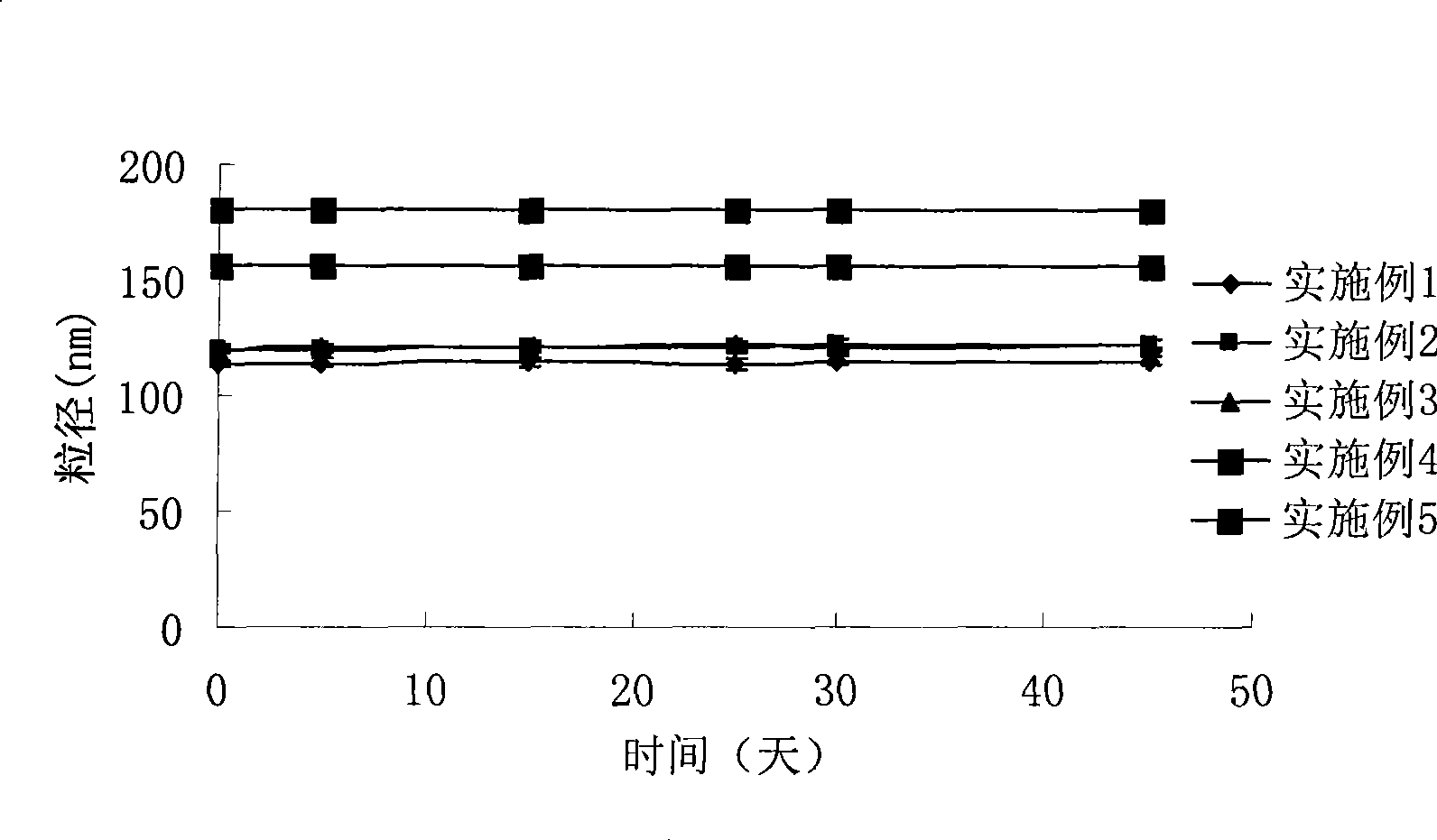
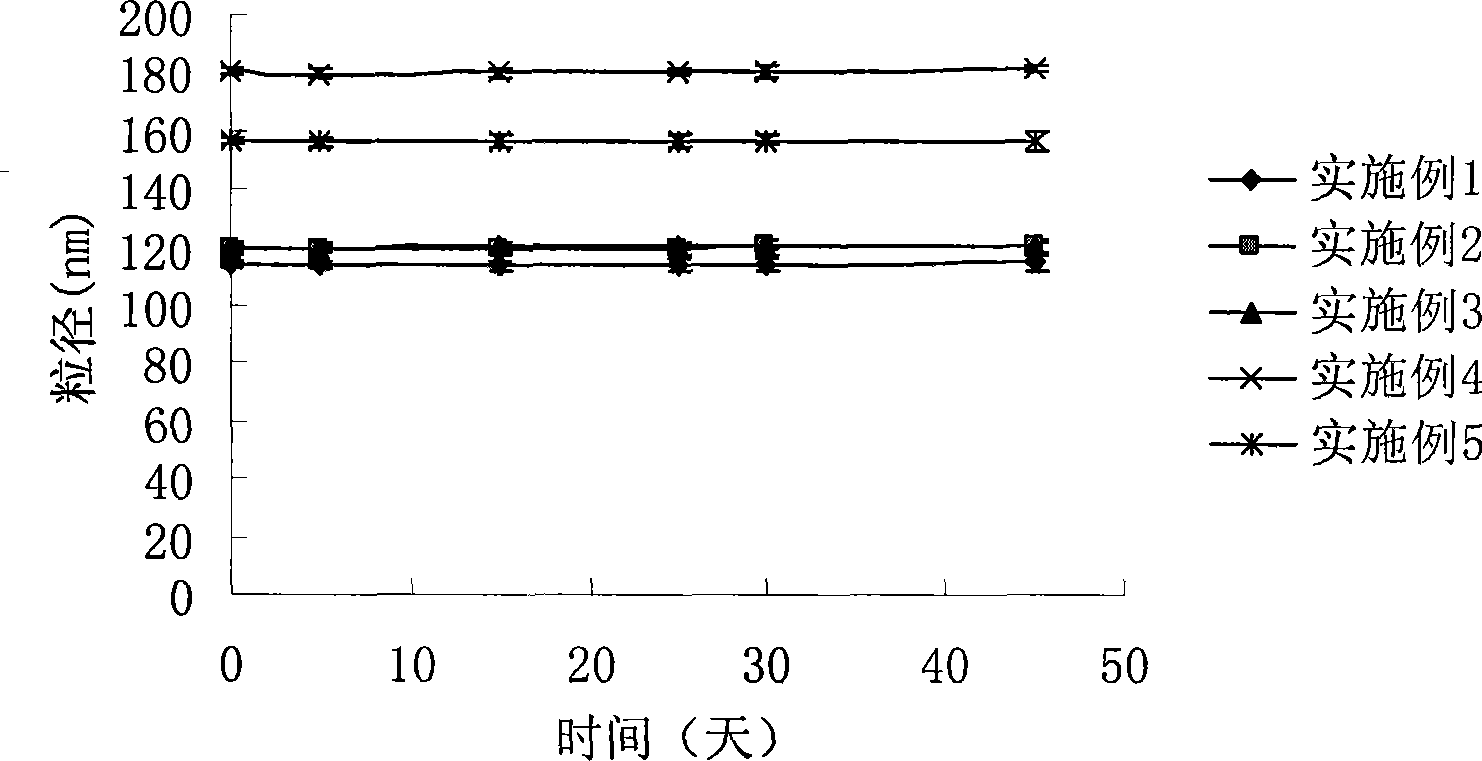
Sex hormone micro-nano human body injection emulsion containing TPGS, and preparation method thereof
InactiveCN103585103AReduce contentAvoid influencePharmaceutical non-active ingredientsEmulsion deliveryMicro nanoMicroorganism
The invention belongs to the field of bioscience and bio-pharmaceutical technology, and specifically relates to a sex hormone micro-nano human body injection emulsion containing TPGS, and a preparation method thereof. Particle size of 90% particles in the sex hormone micro-nano human body injection emulsion is no more than 200nm, and average particle size of the sex hormone micro-nano human body injection emulsion is no more than 180nm, and sex hormone and TPGS are the effective components of the sex hormone micro-nano human body injection emulsion. Beneficial effects of the sex hormone micro-nano human body injection emulsion are that: the sex hormone micro-nano human body injection emulsion contains no obvious lipid components, and is capable of reducing occurrence rate of hyperlipidaemia and microorganism infection; tween is replaced by TPGS, so that influences on intravenous injection caused by antihypertensive effect and slight hemolysis effect of tween are avoided, and cardiovascular diseases induced by tween are avoided; and TPGS comprises hydrophilic PEG chains and hydrophobic vitamin E chains, and the hydrophobic vitamin E chains are capable of inserting into hydrophobic layers of sex hormone, so as to obtain sex hormone preparations coated by PEG, and increase stability of sex hormone.
Owner:杭州伟智生物医药科技有限公司
Arbidol hydrochloride injection emulsion and preparation method thereof
ActiveCN112933043AImprove solubilityImprove stabilityOrganic active ingredientsAntiviralsInjection emulsionIrritation
The invention relates to the technical field of medicines, in particular to an arbidol hydrochloride injection emulsion and a preparation method thereof. The arbidol hydrochloride injection emulsion comprises the following components: arbidol hydrochloride, an emulsifier, a co-emulsifier, oil for injection, an isoosmotic adjusting agent, a pH adjusting agent, an antioxidant and water for injection, wherein the emulsifier is soybean lecithin and Tween 80, and the co-emulsifier is Arabic gum and n-propyl alcohol. According to the preparation method of the arbidol hydrochloride injection emulsion provided by the invention, a high-pressure homogenization process is adopted, and the drug is entrapped in the emulsion droplets, so that the drug loading capacity of the preparation is improved, the solubility and bioavailability of arbidol hydrochloride are improved, the administration frequency is reduced, the toxic and side effects and irritation are reduced, and the preparation is high in stability. The problem that the medicine is easy to separate out in the storage and use processes of the conventional injection emulsion is solved; and meanwhile, administration is convenient, effect taking is fast, the preparation technology is simple, and the preparation method can be used for industrial large-scale production.
Owner:SHIJIAZHUANG NO 4 PHARMA
Pharmaceutical composition compounded with compound liposoluble vitamins and preparation method thereof
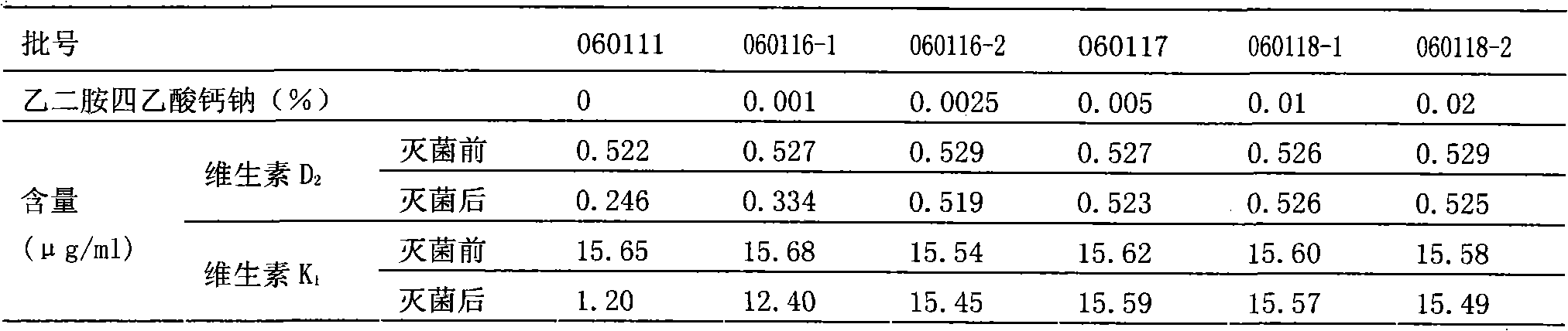
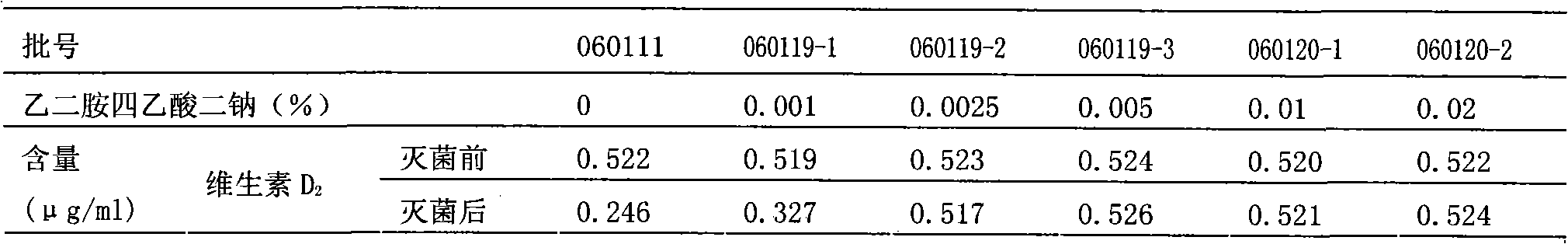
The invention relates to a pharmaceutical composition of compound liposoluble vitamins, comprising vitamin A, vitamin D2, vitamin E, vitamin K1 and a metal ion chelant (such as edetate). The pharmaceutical composition can be made into an injection emulsion, preferably solve the problem that degradation products are generated because the active constituents ( such as the vitamin D2 and the vitamin K1) are oxidized in the preparation and sterilization processes, and ensure the safety of clinical administration.
Owner:SICHUAN KELUN PHARMA CO LTD
Preparation of fotemustine fat emulsion for injection
InactiveCN102085184AGood anti-tumor effectWith sustained releaseOrganic active ingredientsMetabolism disorderSide effectHigh pressure
The invention relates to a preparation method for a fotemustine fat emulsion injection. The invention comprises the following steps: putting soybean oil, mid-chain oil, and oleic acid into a container, stirring the mixture to prepare an oil phase; adding a certain amount of water into a mixture of fotemustine, soybean phosphatide, and glycerin to prepare a water phase; adding the oil phase into the water phase, stirring uniformly, performing high-speed shearing, adjusting the pH to be neutral, homogenizing the mixture by a high-pressure homogenizer to prepare a drug-loaded fat emulsion, filtering, filling into an ampoule, injecting nitrogen, performing melt sealing and autoclaving. The injection emulsion of the invention has the advantages of high efficiency, large drug loading amount, no toxic and side effects, good stability, and convenient usage, and the preparation method is rapid and simple, and is applicable to large amount preparation and industrial production.
Owner:刘振平
Determination method of edetate disodium in clevidipine butyrate injection emulsion
The invention provides a preparation method of a sample used for HPLC detection of disodium edetate in a clevidipine butyrate injection emulsion. The preparation method comprises the following steps: adding an isopropanol and n-hexane mixed liquid into the clevidipine butyrate injection emulsion, shaking the mixed liquid and the emulsion, centrifuging the obtained solution, taking obtained water phase, and adding a metal ion salt solution able to complex edetic acid to form a complex in order to form a sample solution. The invention also provides a method for detecting disodium edetate in the clevidipine butyrate injection emulsion. The detection method is an HPLC method. An oil phase and a water phase in the clevidipine butyrate injection emulsion are effectively separated to make disodium edetate dissolved in the water phase be accurately detected. The detection method has the advantages of high detection sensitivity, good precision and high specificity, and is an effective method for strictly controlling the content of disodium edetate in the clevidipine butyrate injection emulsion.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com