Composition containing antifungal drug and lactate buffering agent
A composition and lactate technology, applied in the directions of antifungal agents, drug delivery, medical preparations of inactive ingredients, etc., can solve problems such as insufficient stability, insufficient stability, and instability of pharmaceutical compositions, and achieve improved chemical stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Prepare the caspofungin composition 1 according to the prescription in the table, and the method is as follows:
[0029]
[0030]
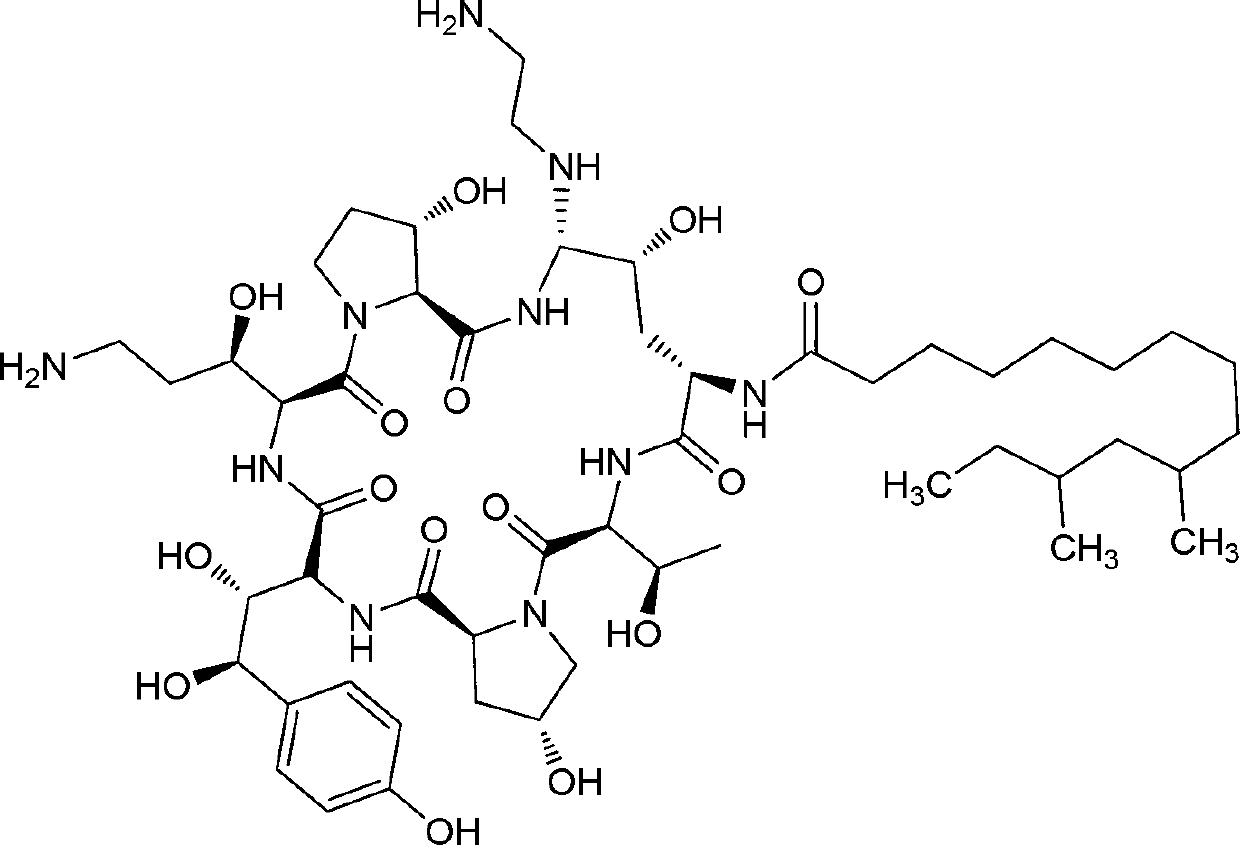
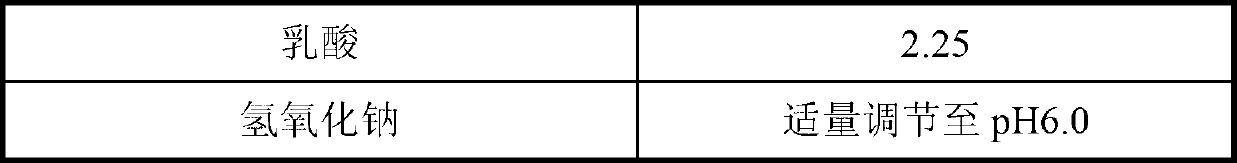
[0031] Add 2g of mannitol and 3g of sucrose into a 100ml volumetric flask, add about 60ml of water, dissolve and add 10mL of 22.50mg / mL lactic acid solution and mix well, then add 4.66g of caspofungin acetate (equivalent to 4.2g caspofungin), shake gently to dissolve and avoid foaming. Then use 1 mol / mL sodium hydroxide to adjust the pH of the solution to 6.0. Then add water to a final volume of 100 mL. After filtering with a 0.22um microporous membrane, it was divided into 10mL vials at a rate of 1.3mL / vial, and freeze-dried to prepare a freeze-dried cake of caspofungin acetate.
Embodiment 2
[0033] Preparation of caspofungin acetate freeze-dried powder with different pH values and comparison at 30°C and 25°C
[0034] Compositions 2, 3 and 4 with pH 5.0, 7.0 and 8.0 were prepared in the same manner as in Example 1.
[0035] The prepared freeze-dried powder was kept as a sample at 30°C for investigation, and samples were taken at 5 days and 10 days respectively, and at 25°C for inspection, and samples were taken at 6 months, and the samples were analyzed and determined by HPLC. , comparing the changes of impurity L-747969 and total impurities, wherein impurity L-747969 is the main hydrolysis and thermal degradation impurity of caspofungin acetate. The results are shown in the table below:
[0036]
[0037]
[0038] From the comparison results of the stability of caspofungin acetate freeze-dried powder with different pH values, it can be seen that within the range of pH 5.0-8.0, the stability of the composition is basically the same, with little difference. ...
Embodiment 3
[0040] Compositions 5, 6, 7, 8, 9, and 10 were prepared according to the method of Example 1. The ingredients and dosage of each composition are shown in the table below, wherein the pH value of the composition is adjusted with NaOH, and the dosage unit of each component is mg / ml.
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com