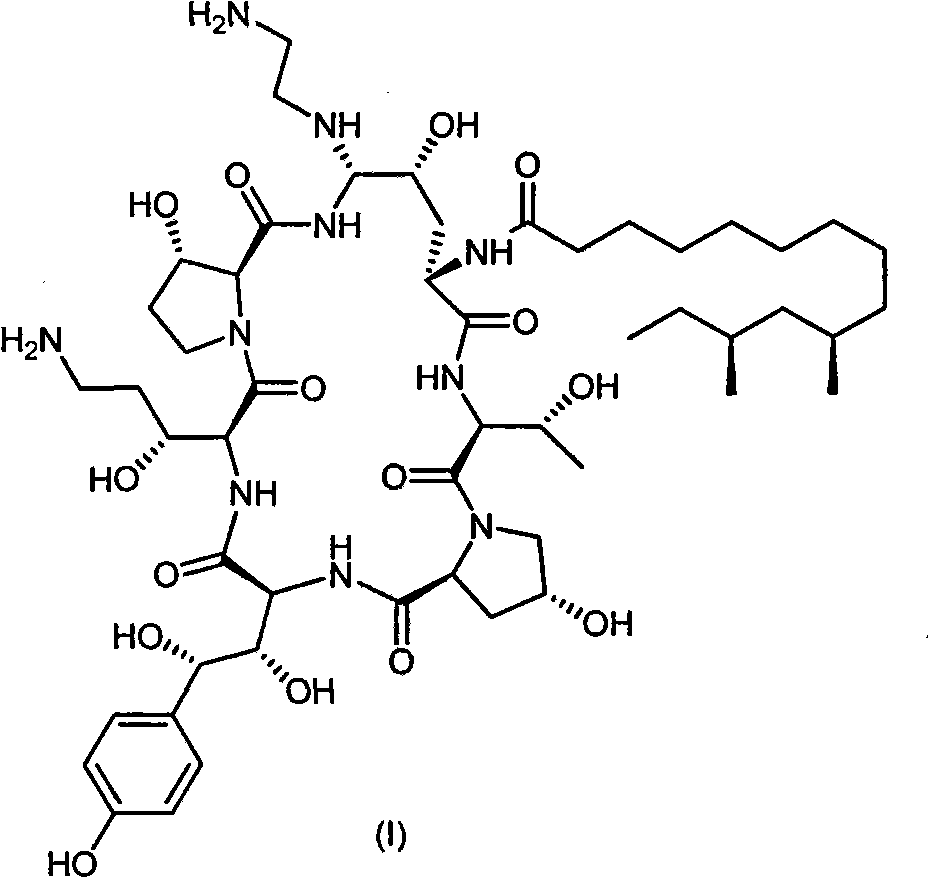
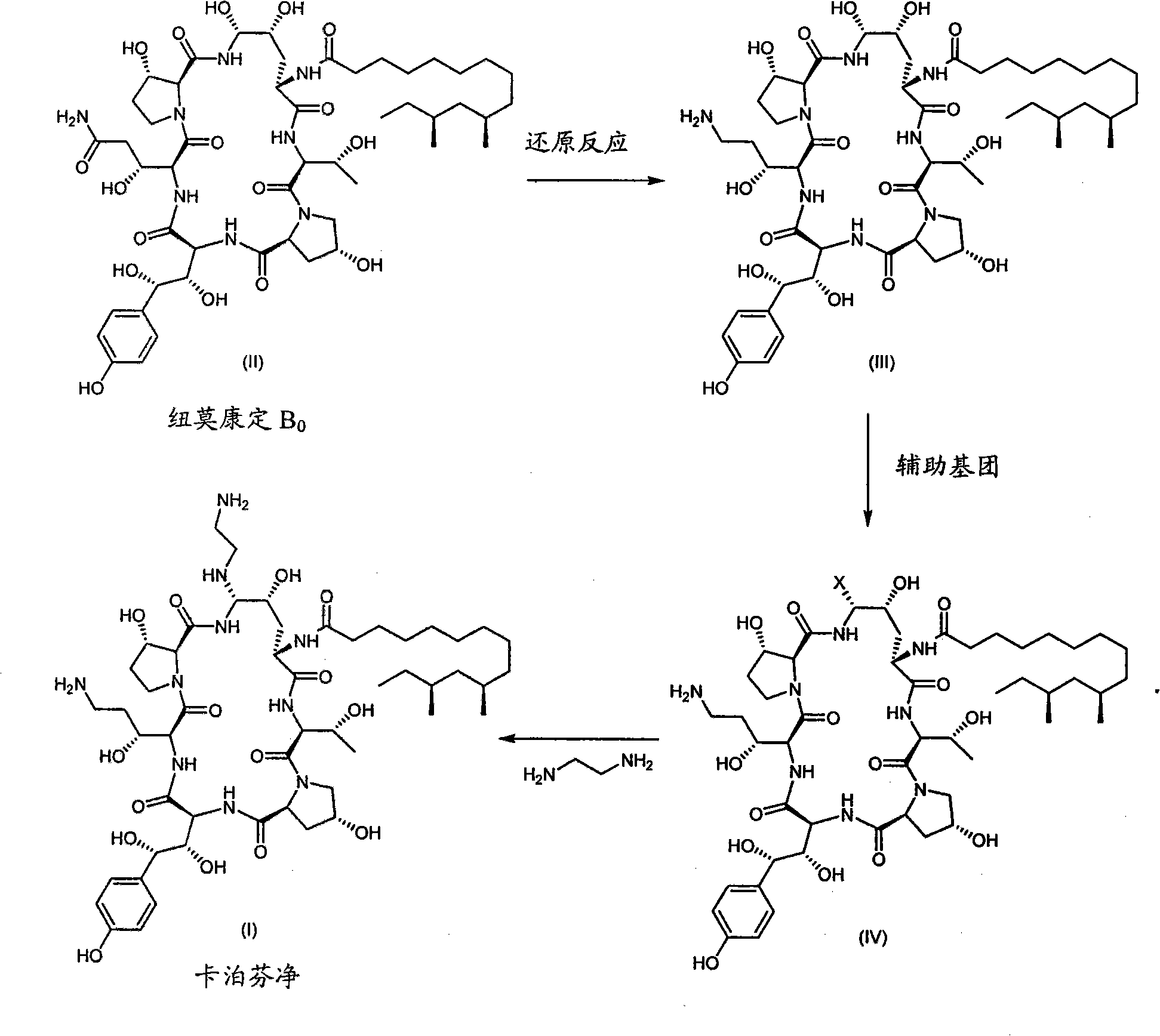
Process for preparing caspofungin and intermediates thereof
一种化合物、分子式的技术,应用在抗真菌剂、肽等方向,能够解决材料损耗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0073] The present invention will now be described in detail with reference to examples, but the examples should not be construed as limiting the scope of the present invention and the appended claims.
[0074] High performance liquid chromatography (HPLC) analysis is carried out under the following conditions: chromatographic column Waters Symmetry C18, 250x 4.6mm, 5μm; column temperature: 45°C; mobile phase: solution A: 0.1% v / v perchloric acid aqueous solution, solution B: acetonitrile, elution gradient 67 / 33A:B to 35 / 65A:B; flow rate: 1.5mL / min ; Detection: 205nm; Integration setting: peak area %; Solution: acetonitrile / water 1:1. Mass spectra were acquired under electrospray ionization conditions, with the device operating in positive ion mode. Analytes are detected in protonated form.
example 1
[0076] A compound of formula VIII, R 1 =-(CO)NH 2 ,R 2 =R 3 =H
[0077] Neomocontin B 0 (2.02 g (grams), 1.90 mmol (mmol)) and trifluoromethanesulfonic acid (3.0 mL (milliliter), 5.09 g, 33.9 mmol) were dissolved in pyridine (30 mL). The mixture was heated to 80 °C and stirred under an inert atmosphere for 12 h (hours). After cooling to 0°C, 1,2-ethylenediamine (1.00 mL, 0.90 g, 15.0 mmol) was added and the mixture was stirred overnight. The reaction was cooled by adding the reaction mixture to a mixture of water (100 mL) and acetic acid (21 mL, 22.0 g, 0.37 mol) to give a solution at pH 5.0. The solution was loaded into a chromatographic column and eluted with a gradient of 20% acetonitrile / 80% water to 25% acetonitrile / 75% water. Evaporation of the organic solvent and lyophilization of the rich cuts afforded 534 mg (22% yield) of the title compound as the triflic acid addition salt. HPLC purity: 87.8%.
[0078] 1 H NMR (nuclear magnetic resonance) (6...
example 2
[0080] A compound of formula VIII, R 1 =-(CO)NH 2 ,R 2 =R 3 =H
[0081] Neomercantin B 0 (100 mg (milligram), 0.094 mmol) was dissolved in pyridine (2 mL), and triethylsilyl triflate (107 μL (microliter), 125 mg, 0.47 mmol) was added. The mixture was stirred at 80 °C for 12 h and the progress of the reaction was monitored by HPLC. After stirring overnight, HPLC showed 72% conversion into new compounds (compound VI, R 1 =-(CO)NH 2 ). LC-MS (liquid chromatography-mass spectrometry) showed m / z 1126.4 as the major product, which confirmed the pyridine substitution. The mixture was cooled to room temperature, and 1,2-ethylenediamine (2 mL) was added. After 15 minutes HPLC showed complete conversion of the pyridine addition to another product with LC-MS mass to nucleus ratio m / z of 1107.6 Å, the predicted mass of the title compound. No separation of the product was performed.
[0082] Example 3
[0083] Compound of formula III
[0084] Neomocontin B ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com