Liquid pharmaceutical composition containing echinocandin antifungal agent caspofungin
A composition and antifungal technology, applied in stabilizers, can solve problems such as increasing production cost, affecting production efficiency, increasing the risk of drug use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0062] Dissolve 1.20g of trehalose in 3ml of water, then add 7.5μl of glacial acetic acid, adjust the pH to 5.1 with 1M sodium hydroxide, then add 0.223g of caspofungin acetate, stir gently to dissolve, and oxidize with 1M hydroxide Adjust the pH to 6.0 with sodium, add water to constant volume to 5mL, and filter through a 0.22 μm membrane. The composition of the composition (Formulation 2) is as follows:
[0063] Caspofungin acetate (by base) 40mg / ml
[0064] Trehalose 240mg / ml
[0065] Glacial acetic acid 1.5mg / ml
[0066] Sodium hydroxide Adjust to pH6.0
[0067] The prepared solution was divided into 2mL antibiotic bottles according to 0.5mL / bottle, fully stoppered with rubber stoppers, capped, and placed under the conditions of 40°C, 75%RH and 30°C, 65%RH respectively for stability investigation , and samples were taken for HPLC analysis after 8 weeks.
Embodiment 3
[0069] The preparation process is similar to Example 2, except that in the preparation process, the stabilizing agent is selected between trehalose, sucrose or the composition of trehalose and sucrose, and the pH regulator used is between acetate, phosphate or citric acid Choose between salt, even do not add any additional pH adjuster, thus obtain different formulations, the composition of each composition formulation is as follows:
[0070]
[0071] The compositions of each formula were also subjected to the stability investigation described in Example 2.
Embodiment 4
[0073] After the samples of Comparative Example 1, Example 2 and Example 3 were tested for stability, the active substances were analyzed by HPLC.
[0074] The results of the 40°C stability test are shown in the table below:
[0075] recipe number
[0076] The results of the stability test at 30°C are shown in the table below:
[0077] recipe number
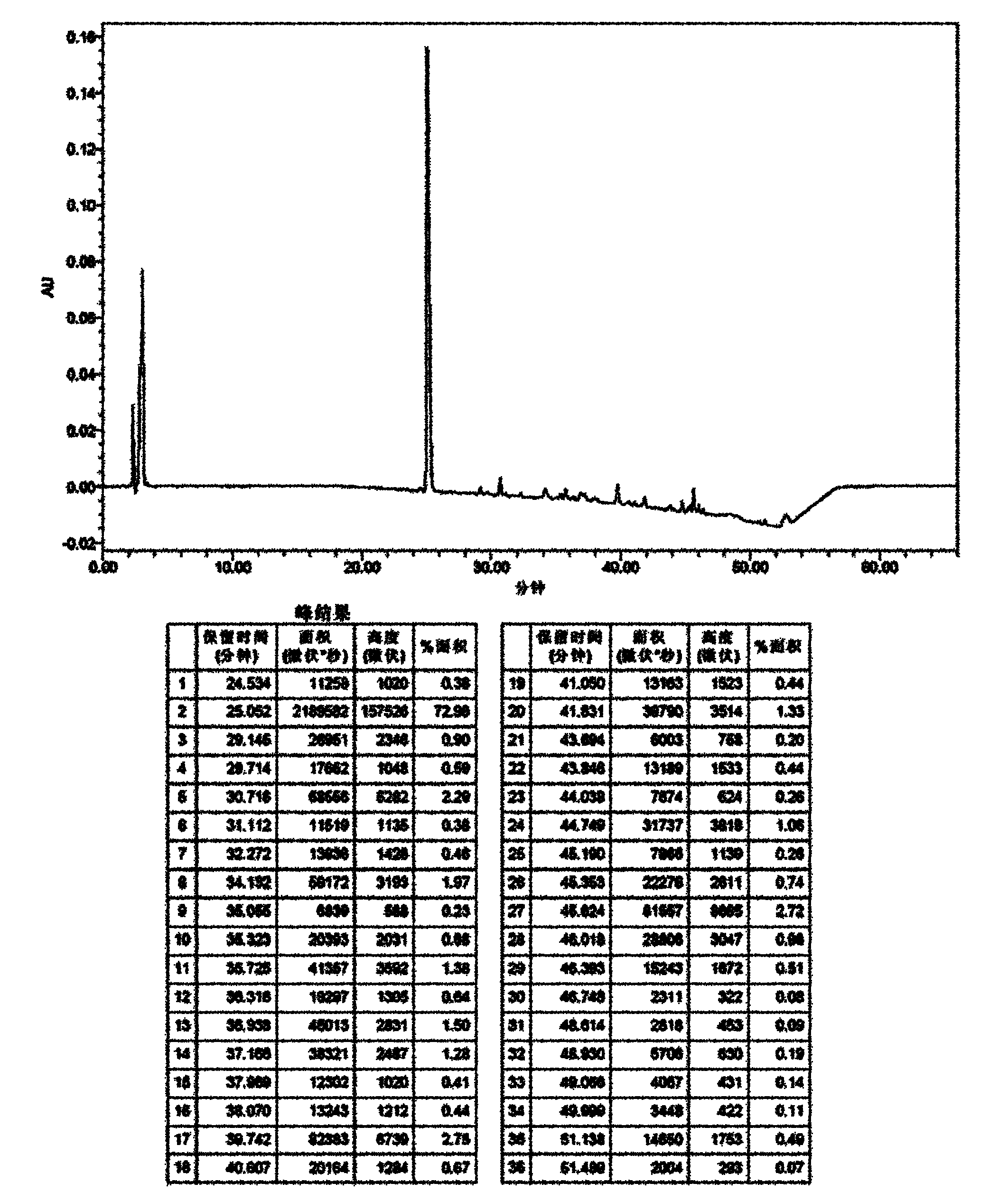
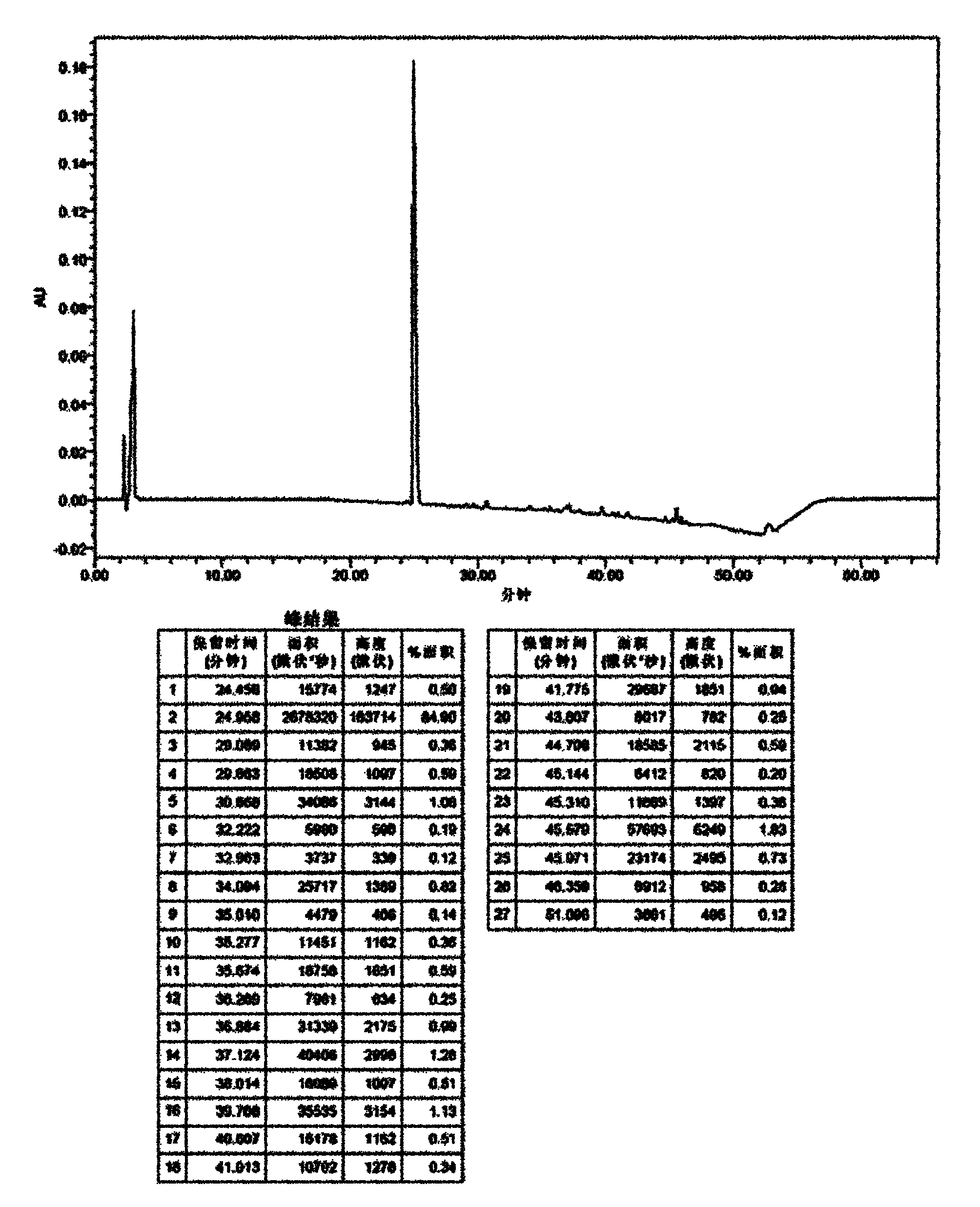
[0078] It can be seen from the data in the above table that the stability of the liquid composition formulation is significantly better than that of the freeze-dried composition of formulation 1, especially when the content of the stabilizer reaches a certain concentration. The HPLC chromatogram after the stability investigation of formula 1 and formula 5 is shown in the attachment Figure 1~4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com